Abstract
Objectives: We examined the prevalence of low testosterone (LT) in the subset of men in the Proscar Long-term Efficacy and Safety Study (PLESS) who had serum total testosterone (TT) measured at baseline.
Methods: PLESS enrolled 3040 men with benign prostatic hyperplasia (BPH). Of these men, 299 had TT and body mass index (BMI) measurements at baseline. Patients were classified as having LT if their baseline TT was <300 ng/dl.
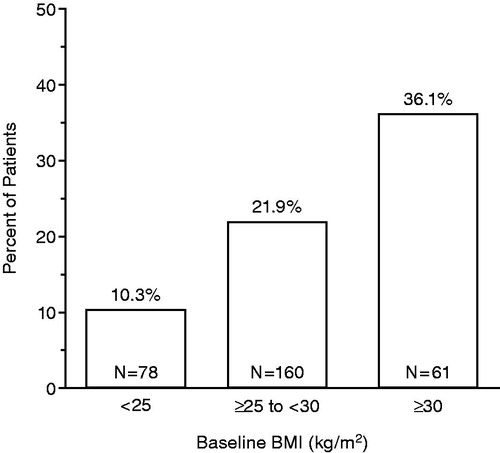
Results: Of the 299 PLESS patients with baseline TT and BMI measurements, 65 (21.7%) had LT. The prevalence of LT increased with increasing BMI, occurring in 8/78 (10.3%) normal weight patients (baseline BMI <25 kg/m2), 35/160 (21.9%) overweight patients (baseline BMI ≥25–<30 kg/m2), and 22/61 (36.1%) obese patients (baseline BMI ≥30 kg/m2).
Conclusions: LT was observed in more than one in five PLESS patients with baseline TT and BMI measurements. The prevalence of LT increased with increasing BMI – more than one in three obese PLESS patients with baseline TT measurements had LT.
Introduction
Increased body mass index (BMI) is a shared risk factor for benign prostatic hyperplasia (BPH) and low testosterone (LT) in aging men [Citation1–3]. Few studies have examined the prevalence of LT in aging men with BPH. A previous study in 312 aging Austrian men with BPH (mean age: 63 years) reported that more than one in five patients had LT, defined as a total serum testosterone (TT) level of <300 ng/dl [Citation4].
The Proscar Long-term Efficacy and Safety Study (PLESS) was a 4 year, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of finasteride 5 mg in 3040 men aged 45–78 with symptomatic BPH, enlarged prostates and no evidence of prostate cancer [Citation5]. TT was measured in a randomly selected subset of 10% of the patient population. We examined the prevalence of LT, defined as a baseline TT <300 ng/dl, in these men.
Methods
Details concerning the study design for PLESS, conducted between 1991 and 1996, have been published previously [Citation5]. Briefly, the patient entry criteria included an enlarged prostate by digital rectal examination, moderate to severe symptoms of urinary obstruction, decreased maximal urinary flow rate (<15 ml/s with a voided volume of 150 ml or more), no concurrent use of α-blockers or antiandrogens, no prior prostate surgery, no history of prostatitis or recurrent urinary tract infections, a prostate-specific antigen level of <10 ng/ml, and no evidence of prostate or bladder cancer. A total of 3040 men participated in the trial. Of these 3040 men, a randomly selected subset of 301 had TT measured at baseline. Of these 301 men, 299 had both TT and BMI measurements at baseline; these data were examined in the present post hoc analysis. Blood samples for TT measurement were drawn between 8 am and 12 noon in a nonstandardized manner (i.e. whenever the patient was in the clinic for his visit). TT levels were determined by radioimmunoassay at Endocrine Sciences (Calabasas Hills, CA).
In the present post hoc analysis, men with LT were defined as those with baseline TT <300 ng/dl (i.e. lower limit of eugonadal reference range for young men) [Citation6]. Patients were grouped according to BMI categories, <25 kg/m2 (normal weight), ≥25–<30 kg/m2 (overweight) or ≥30 kg/m2 (obese), and the percentage of patients with LT in each BMI group was evaluated.
Results
Patient characteristics
Baseline characteristics for the 299 patients who had TT and BMI measured at baseline and who were included in the present analysis are shown in , by BMI subgroup. Within this overall analysis cohort, mean ± SD baseline TT was 424.6 ± 154.3 ng/dl, mean ± SD BMI was 27.4 ± 3.9 kg/m2, mean ± SD age was 63.4 ± 6.8 years, 26.1% had normal body weight, 53.5% were overweight and 20.4% were obese. For the cohort who had LT (baseline TT < 300 ng/dl), mean ± SD baseline TT was 260.9 ± 33.8 ng/dl, mean ± SD BMI was 29.0 ± 4.1 kg/m2 and mean ± SD age was 63.3 ± 6.2 years. For the cohort with normal baseline TT (baseline TT ≥ 300 ng/dl), mean ± SD baseline TT was 470.1 ± 143.6 ng/dl, mean ± SD BMI was 27.0 ± 3.8 kg/m2 and mean ± SD age was 63.4 ± 7.0 years. There was a slight decrease in mean age with increasing BMI (mean age was 65.2, 63.0 and 62.1 years in the <25, ≥25–<30 and ≥30 kg/m2 BMI subgroups, respectively).
Table 1. Baseline characteristics.
Prevalence of LT
Of the 299 patients with baseline TT and BMI measurements, 65 (21.7%) had LT. In the subgroup of men with baseline BMI <25 kg/m2 (normal weight), 8/78 (10.3%) had LT, while 35/160 (21.9%) and 22/61 (36.1%) had LT in the subgroups with baseline BMI ≥25 to <30 kg/m2 (overweight) and ≥30 kg/m2 (obese), respectively (). Of the patients included in this analysis, 74% (221/299 patients) were overweight or obese. Among the 65 patients with LT, 88% were overweight or obese.
Discussion
The prevalence of LT observed in the present analysis of baseline TT and BMI data from PLESS (65/299 patients, or 21.7%) was similar to that reported in previous analyses evaluating hypogonadism in BPH patients [Citation4] and was within the broad range of LT prevalence reported across the general population of aging males [Citation7–9]. The results of this analysis are also in agreement with previous studies in which LT was observed to be associated with increased BMI [Citation10–15]. As expected given the well-established positive relationship between the prevalence and severity of BPH and increased BMI [Citation1–3], the majority (74%) of patients included in the present analysis were overweight or obese, and most (88%) of the patients with LT were overweight or obese.
There are a number of mechanisms that may explain the inverse relationship between higher BMI and testosterone levels in men. In men, adipose tissue is the main peripheral source of aromatase, which catalyzes the irreversible conversion of T to estradiol. The increased adiposity in obesity may be associated with increased aromatase activity, which could lead to an increase in the conversion of T to estradiol [Citation16]. Obese men have been shown to have elevated serum estradiol levels [Citation17]. Increased estradiol levels may, in turn, cause pituitary suppression [Citation16], which may explain, at least in part, the reduction in gonadotropin release reported in obese men [Citation16,Citation17]. Obesity has also been shown to be associated with reduced sex hormone-binding globulin levels, which could lead to a further reduction in serum testosterone levels [Citation17,Citation18].
In light of the role testosterone plays in maintaining male sexual function [Citation19,Citation20], together with its roles in regulation of bone density, muscle mass and function, fat mass and cardiovascular fitness [Citation21,Citation22], physicians should be aware of the relatively high prevalence (36%) of LT in obese men with BPH observed in this study. Consideration might also be given to the possibility that symptoms associated with LT could exacerbate the general deterioration in quality of life associated with BPH [Citation23,Citation24].
The most widely prescribed treatment for men with symptomatic LT is testosterone replacement therapy. However, significant increases in prostate volume and PSA levels have been reported in some hypogonadal patients receiving testosterone therapy [Citation25–29]. Prostate volume and serum PSA are positively correlated with the risk of the serious urinary outcomes of acute urinary retention and BPH-related surgery in men with BPH [Citation30,Citation31]. Moreover, pharmacologically induced decreases in prostate volume and PSA have been shown to be associated with reductions in the risk of acute urinary retention and BPH-related surgery in BPH patients [Citation5,Citation32,Citation33]. Taken together, these findings raise an obvious concern as to whether some BPH patients with LT who may experience significant testosterone-induced increases in prostate volume and/or PSA would be at risk of a further increase in their already elevated likelihood of developing acute urinary retention or needing BPH-related surgery. To date, no large, long-term, randomized and controlled studies have examined the effect of testosterone therapy on urinary outcome risk in BPH patients with LT. Additionally, testosterone therapies that cause large elevations in serum PSA [Citation25] in some hypogonadal patients could also complicate use of serum PSA as part of screening for prostate cancer, including complicating the interpretation of serum PSA levels in BPH patients treated with 5α-reductase inhibitors. Until prospective studies are done to address these potential areas of concern, it would appear prudent for physicians to monitor urinary function in the subgroup of BPH patients with LT who may experience significant prostate tissue stimulation while receiving testosterone therapy.
In spite of its well-known stimulatory effect on prostate tissue, recent studies have suggested that testosterone therapy can actually improve urinary symptoms in hypogonadal BPH patients. The mechanism(s) by which testosterone produces this apparently paradoxical beneficial effect is not clear [Citation34,Citation35]. Given the important role of androgens in male urinogenital physiology, it is conceivable that the re-normalization of serum and intraprostatic androgen levels with testosterone therapy could lead to re-normalization of intraprostatic architecture and function in hypogonadal BPH patients. Further studies are needed to investigate the effects of testosterone therapy on prostatic function and urinary symptoms in these patients.
Conclusions
In conclusion, this analysis of baseline data from 299 BPH patients participating in PLESS demonstrated that, in these men, LT occurred in more than one out of every five patients. The prevalence of LT was highest (36%) among obese BPH patients. Practitioners concerned with managing aging men with BPH should be mindful of the prevalence of LT in these patients, which may be associated with deleterious effects on muscle mass/strength, bone, fat mass, sexual function and cardio-metabolic risk. Further studies are needed to determine whether testosterone therapy could lead to clinical benefits in BPH patients.
Declaration of interest
PLESS was funded by Merck & Co., Inc. Authors O’Neill, Lowe, Hanson and Meehan are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Whitehouse Station, NJ. Author Kaplan is a PLESS investigator and an employee of Weill Cornell Medical College, New York, NY.
Acknowledgements
The authors wish to acknowledge the contributions of the many investigators, study coordinators and patients who contributed to PLESS. Editorial support was provided by Kathleen Newcomb (Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ).
References
- Giovannucci E, Rimm EB, Chute CG, et al. Obesity and benign prostatic hyperplasia. Am J Epidemiol 1994;140:989–1002
- Lee RK, Chung D, Chughtai B, et al. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int 2012;110:540–5
- Wang S, Mao Q, Lin Y, et al. Body mass index and risk of BPH: a meta-analysis. Prostate Cancer Prostatic Dis 2012;15:265–72
- Schatzl G, Brossner C, Schmid S, et al. Endocrine status in elderly men with lower urinary tract symptoms: correlation of age, hormonal status, and lower urinary tract function. The Prostate Study Group of the Austrian Society of Urology. Urology 2000;55:397–402
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003;349:2387–98
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536–59
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007;92:4241–7
- Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2001;86:724–31
- Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006;60:762–9
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–9
- Kaplan SA, Meehan AG, Shah A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively high incidence of erectile dysfunction observed in these men? J Urol 2006;176:1524–7
- Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004;27:1036–41
- Laaksonen DE, Niskanen L, Punnonen K, et al. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. J Clin Endocrinol Metab 2005;90:712–9
- Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci 2002;57:M76–99
- Roehrborn CG, Lee M, Meehan A, Waldstreicher J. Effects of finasteride on serum testosterone and body mass index in men with benign prostatic hyperplasia. Urology 2003;62:894–9
- Cohen PG. The hypogonadal-obesity cycle: role of aromatase in modulating the testosterone-estradiol shunt – a major factor in the genesis of morbid obesity. Med Hypotheses 1999;52:49–51
- Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab 1993;76:1140–6
- Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J Clin Endocrinol Metab 1996;81:1821–6
- Aversa A, Isidori AM, De Martino MU, et al. Androgens and penile erection: evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunction. Clin Endocrinol (Oxf) 2000;53:517–22
- Foresta C, Caretta N, Rossato M, et al. Role of androgens in erectile function. J Urol 2004;171:2358–62, quiz
- Makhsida N, Shah J, Yan G, et al. Hypogonadism and metabolic syndrome: implications for testosterone therapy. J Urol 2005;174:827–34
- Gooren LJ. The age-related decline of androgen levels in men: clinically significant? Br J Urol 1996;78:763–8
- Roberts RO, Jacobsen SJ, Rhodes T, et al. Natural history of prostatism: impaired health states in men with lower urinary tract symptoms. J Urol 1997;157:1711–7
- Girman CJ, Jacobsen SJ, Tsukamoto T, et al. Health-related quality of life associated with lower urinary tract symptoms in four countries. Urology 1998;51:428–36
- U.S. prescribing information for ANDROGEL® (testosteron gel), April 2011. FDA. 4-1-2011. 8-16-2012
- Behre HM, Bohmeyer J, Nieschlag E. Prostate volume in testosterone-treated and untreated hypogonadal men in comparison to age-matched normal controls. Clin Endocrinol (Oxf) 1994;40:341–9
- Marks LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA 2006;296:2351–61
- Rhoden EL and Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004;350:482–92
- Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E. Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic study. J Clin Endocrinol Metab 2003;88:2049–54
- Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 1999;53:473–80
- Roehrborn CG, Malice M, Cook TJ, Girman CJ. Clinical predictors of spontaneous acute urinary retention in men with LUTS and clinical BPH: a comprehensive analysis of the pooled placebo groups of several large clinical trials. Urology 2001;58:210–6
- McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med 1998;338:557–63
- Roehrborn CG, Boyle P, Nickel JC, et al. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002;60:434–41
- Kalinchenko S, Vishnevskiy EL, Koval AN, et al. Beneficial effects of testosterone administration on symptoms of the lower urinary tract in men with late-onset hypogonadism: a pilot study. Aging Male 2008;11:57–61
- Shigehara K, Sugimoto K, Konaka H, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male 2011;14:53–8