Abstract
Aim: The purpose of this study was to evaluate the effect of parathyroid hormone (PTH) (1–84) in a model of male osteoporosis induced by orchidectomy in rats.
Methods: Six-month-old Wistar rats were used as follows: SHAM (simulated orchidectomy), orchidectomized (ORX), ORX + PTH1 (ORX and treated with 10 µg/Kg/d of PTH 1–84) and ORX + PTH2 (ORX and treated with 50 µg/Kg/d of PTH 1–84) over 3 months, with treatment beginning three months after orchidectomy.
Results: Orchidectomy resulted in a decreased of femoral and lumbar bone mineral density (BMD), a worsening of trabecular and cortical microarchitecture and a decrease in biomechanical properties. Both doses of PTH (1–84) partially (low dose) or totally (high dose) restored the ORX-induced changes. Serum C-telopeptide of type I collagen/5b isoenzyme of tartrate-resistant acid phosphatase (CTX/TRAP) resorption index increased after orchidectomy. Osteocalcin (bone Gla protein; BGP) levels were not affected by orchidectomy. PTH (1–84) treatment did not produce any changes in the levels of CTX/TRAP with respect to the ORX group. BGP levels increased with PTH treatment.
Conclusion: PTH (1–84) is able to restore the adverse effects of orchidectomy on bone as measured by BMD, microstructural and biomechanical properties and bone remodeling markers.
Introduction
Osteoporosis is defined as a decrease in bone mass with alterations in bone microarchitecture, facilitating the appearance of fractures. It is well-known that osteoporosis-related fractures (low-trauma or fragility fractures) cause substantial disability, health-related costs and mortality among postmenopausal women and older men. Despite the fact that male osteoporosis is less prevalent than postmenopausal osteoporosis, Dawson-Hughes et al. [Citation1] found that in the National Health and Nutrition Examination Survey (NHANES III) population, 41% of US Caucasian post-menopausal women and 22% of men aged 50 years and older met the criteria for treatment of osteoporosis. One of the most important causes of osteoporosis in men seems to be a change in the level of sex steroid hormones with increasing age. The testosterone deficiency-induced osteoporosis in men develops later in life than postmenopausal osteoporosis in women, but the morbidity and mortality after osteoporotic fractures are greater [Citation2]. The main effects of sex-hormone deficiency are increase of the volume of reabsorbed bone versus reduced the volume of formed bone, with a total negative balance in bone mass [Citation3].
According to the physiopathology and the mechanism of the disease, there are several options for the treatment of osteoporosis, which fall under the headings of anticatabolic, anabolic (bone-forming) or mixed agents. Hence, despite comparable antifracture efficacy, all these agents are likely to modify the various determinants of bone strength in very different ways. The current paradigm of a bone-forming agent is parathyroid hormone (PTH). PTH is used as anabolic treatment, increasing bone formation and thus bone mass, although it also stimulates bone remodeling as part of its action [Citation4]. Moreover, the positive effect of PTH on post-menopausal osteoporotic bone has led researchers to use the agent also for treatment of male osteoporosis [Citation5]. It has been described that microstructural alterations, such as loss of trabecular connectivity, are implicated in the increased propensity for fracture. Recent two-dimensional (2D) and three-dimensional (3D) assessments of cancellous bone structure have shown that PTH can re-establish lost trabecular connectivity in animals and humans [Citation6]. Two forms of PTH are licensed in Europe for the treatment of severe osteoporosis: the full-length form, PTH (1–84), analogous to the human hormone containing 84 residues and a truncated version of the full-length protein representing a 34-residue N-terminal domain, PTH (1–34) (teriparatide). No clear pronouncements can be made about the potential differences in effectiveness and safety between PTH (1–34) and PTH (1–84); but with regard to the efficacy, a convincing reduction of vertebral fractures was shown in both cases [Citation7,Citation8].
During the past decade, most in vivo studies of PTH pharmacological actions have used rats. The rat skeleton has proved useful in understanding alterations in modeling drift, bone growth and resorption processes, and as a general predictor of the effect of pharmacological agents on bone mass in humans. The experimental model of osteoporosis induced by gonadectomy in rats has been widely used to study the effect of different antiosteoporotic agents, both in male and female animals [Citation9]. In addition, the microarchitectural properties of bone and biomechanical data obtained from animal specimens, could lead us to understand the underlying mechanisms of action of the drugs, without raising the same ethical or methodological issues at hand when using human bone.
Regarding the potency of PTH peptides, teriparatide is thought to be similar to the natural cleavage product; also teriparatide retained the bioactivity of PTH bioassay [Citation10] and has been shown to increase bone mass equivalently in ovariectomized (OVX) and orchidectomized (ORX) young rats [Citation11,Citation12]. Studies have been carried out to compare the effects of these two peptides in bone of OVX rats; experimental works have been done showing no significant biological differences between PTH (1–34) and PTH (1–84) [Citation13–15]. However, to our knowledge, no studies have been published on the effect of PTH (1–84) in ORX rats. The purpose of this study is to assess the effects of PTH (1–84) on an experimentally validated model of male osteoporosis induced by orchidectomy in rats. To this end, we first assessed the changes in bone mass as determined by dual-energy X-ray absorptiometry (DEXA), bone microarchitecture quantified by microcomputed tomography and bone strength by biomechanical testing in the femur and lumbar spine of rats that overcome surgical gonadectomy. Second, we identified how these changes were reversed with the administration of PTH (1–84). Bone remodeling variations were also analyzed through serum biochemical markers, given that bone turnover is directly involved in bone-tissue mineralization and in microfracture repair.
Materials and methods
Animals
Forty-six-month-old male Wistar rats, weighing 445 ± 37 g (mean ± SD), were used. The animals were kept under constant living conditions (22 °C, 12 h per day of light–dark cycles) and food (complete diet for adult rats (maintenance phase, A04), SAFE, Augy, France) and water were available ad libitum.
The animals were randomized into the following groups: one sham-operated group (SHAM; n = 10) and three castrated groups (ORX; n = 30). Orchidectomy was performed in each animal using ketamine (40 mg/kg, Ketolar, Bayer, San Fernando de Henares, Madrid, Spain) and xilacine (8 mg/kg, Rompún, Parke-Davis, Pfizer, Alcobendas, Madrid, Spain). The SHAM and ORX groups received saline; the two treatment groups received PTH (1–84): one group at a daily dose of 10 μg/Kg body weight (ORX + PTH1, n = 10) and the other group was administered a daily dose of 50 μg/Kg body weight (ORX + PTH2, n = 10). Treatment began three months after surgery and was maintained for three months. Both, PTH (1–84) and saline were administered subcutaneously. Recombinant human PTH (1–84) (Preotact©) was kindly supplied by Nycomed Pharma S.A. (Madrid, España). Preotact© in the form of a two-chambers cartridge contains 1.1 ml of solvent and 1.61 mg of lyophilized PTH (1–84). The solvent, containing metacresol and distilled water, was extracted with an insulin syringe and added to the PTH compartment. This mixture was recovered and diluted with saline solution to obtain two solutions of 5 μg/100 μL and 25 μg/100 μL, respectively. ORX + PTH1 and ORX + PTH2 rats were treated with 200 μL of each solution, respectively. On the day following the last treatment, the experimental animals were weighed and sacrificed by exsanguination under ether anesthesia.
Blood samples were obtained by cardiac puncture, and serum samples were immediately frozen at −80 °C as aliquots until determination of biochemical markers of bone turnover. Once the blood was collected, the animals were frozen at −20 °C until determination of bone mineral density (BMD) in previously thawed animals. Prior to BMD analyses, the left femurs were excised and cleaned of adjacent tissue. The right femur was also excised and cleaned for computerized microtomographic analysis (µCT) and biomechanical testing. Lumbar spine BMD was determined in situ. Repeated freeze–thaw cycles have been shown to have no influence on the mechanical properties of bone [Citation16]. All procedures were carried out in accordance with European Community Standards on the Care and Use of Laboratory Animals and after approval of the Ethics Committee of Instituto de Investigación Sanitaria Fundación Jiménez Díaz.
Bone mineral density
BMD was determined in situ in the lumbar spine (L2, L3 and L4) and in the whole left femur by DEXA using a HOLOGIC QDR-1000 TM (S/N 277) (Hologic Inc., Waltham, MA) with small animal software (Walthan, Massachusetts, USA) [Citation17]. Intra-assay and inter-assay variation coefficients were <0.53% and <1.2%, respectively. The scans of the femur were analyzed for BMD of the whole femur. The scans of the ventral-dorsal (VD) plane of L2, L3 and L4 vertebrae were analyzed for lumbar BMD (LBMD).
Trabecular and cortical microarchitecture analysis of femur by micro-CT
The distal region of the right femur was analyzed by µCT (SkyScan N.V., Aartselaar, Belgium), imaged with a X-ray tube voltage of 100 kV and current of 100 μA, and with a 1.0 mm aluminum filter. The scanning angular rotation was 185°, and the angular increment was 0.45°. The voxel size was 11.0 μm and 13.0 μm, respectively. Data sets were reconstructed using a modified Feldkamp algorithm [Citation18] and segmented into binary images (8 bit BMP images) using adaptive local thresholding. For analysis of the microarchitectural properties of trabecular and cortical bone regions, femora specimens were evaluated within a conforming volume of interest (VOI). Both trabecular and cortical bone regions were obtained by free drawing regions of interest and analyzed using the commercial software provided with the equipment (SkyScan™ CT-analyzer software, version 1.7.0).
In the case of the trabecular bone femur region, a VOI was selected starting at a distance of 1.00 mm from the growth plate and extending a further longitudinal distance of 2.50 mm in the proximal direction (226 image slices analyzed). Morphometric indices of trabecular bone region were determined from the microtomographic data sets (integrated over a VOI) using direct 3D morphometry. Total volume of VOI (tissue volume; TV; mm3) and trabecular bone volume (BV; mm3) were calculated based on the hexahedral marching cubes volume model of the VOI. Trabecular bone volume (BV/TV; %) was directly calculated. Trabecular thickness (Tb.Th; mm), trabecular separation (TbS; mm) and trabecular number (Tb.N; 1/mm) were measured directly on 3D images using methods previously described [Citation19,Citation20]. Measurements of Tb.Th were calibrated by scanning and analyzing three aluminum foils with thicknesses of 50, 125 and 250 μm. The non-metric indices, structure model index (SMI) and trabecular bone pattern factor (Tb.Pf; 1/mm) were also calculated using the direct 3D model. The SMI parameter indicates the relative prevalence of rods and plates in a 3D structure [Citation21]. The Tb.Pf measures the relative convexity or concavity of the total bone surface [Citation22]. The degree of anisotropy (DA) represents trabecular anisotropy, defined as the ratio between the maximal and minimal radius of the mean intercept length [Citation23].
Cortical bone parameters were measured in 2D from individual 2D cross-sectional images. A total of 160 slices were analyzed, starting at a distance of 4.00 mm from the growth plate and extending a further longitudinal distance of 1.00 mm. We assessed mean total crossectional bone area (B.Ar; mm2) and mean polar moment of inertia (MMI; mm4), which indicates the resistance to rotation of a cross-section about a chosen axis being the rotational analogue of mass for linear motion. The coefficient of variation values for all these measurements were <5%.
Biomechanical testing
The femora were subjected to mechanical testing with a Microtest EM1/10/FR/m testing machine (Microtest, S.A., Madrid, Spain), and three-point bending strength was measured. Each bone was compressed at the midshaft with a constant speed of 10 mm/min until failure. A load-displacement curve was obtained and used to determine bone structural parameters (extrinsic). The load-displacement curve was normalized and converted into a stress-strain curve to obtain bone material properties (intrinsic) [Citation24]. Whole bone extrinsic biomechanical properties reported include ultimate force (maximum force that the specimen sustained), stiffness (the slope of the linear portion of the load-displacement curve) and work to failure (the area under the load-displacement curve). Bone tissue intrinsic biomechanical properties include ultimate stress, apparent young modulus and toughness, which are independent of cross-sectional size and shape.
Biochemical markers of bone turnover
Serum bone Gla protein (osteocalcin) (BGP) was determined by enzyme-linked immuno sorbent assay (ELISA) for the specific quantitative determination of rat osteocalcin levels (Rat-MID Osteocalcin, IDS, Boldon, UK). The sensitivity of this assay was 50 ng/ml. Intra- and inter-assay coefficients of variation of the method were <5.0% and <6.6%, respectively.
Serum 5b isoenzyme of tartrate-resistant acid phosphatase (TRAP) was measured by an ELISA specific for rat TRAP (RatTRAP Assay, IDS). The sensitivity of the assay was 0.1 U/L. Intra- and inter-assay variation coefficients of the method were <5.0% and <5.5%, respectively.
Serum C-telopeptide of type I collagen (CTX) was measured by an ELISA specific for rat CTX (RatLaps ELISA, IDS). The sensitivity of the assay was 2.0 ng/ml. Intra- and inter-assay variation coefficients of the method were <5.6% and <10.5%, respectively.
Statistical analyses
The results of the experiments were expressed as the mean ± SD of the different parameters. LBMD was expressed as the mean values of L2, L3 and L4 results. A non-parametric method, Mann–Whitney test (Medcalc Software Program, Ostend, Belgium), was used to compare the different treatment groups. A p value <0.05 was accepted as denoting a significant difference.
Results
Bone mineral density
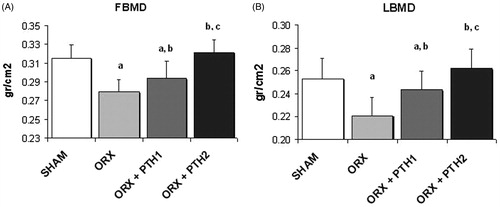
As compared with the SHAM group, femoral BMD (FBMD) and LBMD were significantly decreased in ORX rats by 11% (p < 0.0001) and 13% (p < 0.0001), respectively. Administration of the low dose of PTH (10 μg/Kg/d) to these rats with low bone mass, produced a significant increase in FBMD (p < 0.05) and LBMD (p < 0.005) with respect to the ORX group. Administration of the high dose of PTH (50 μg/Kg/d) restored FBMD (p < 0.001 versus ORX group) and LBMD (p < 0.01 versus ORX group); BMD levels were similar to those of the SHAM group ().
Figure 1. BMD (g/cm2) measured (A) in femur (FBMD) and (B) in lumbar vertebrae (LBMD); six-month-old male Wistar rats at the beginning of the study, distributed into four groups: Sham-operated rats (SHAM), castrated rats (orchidectomy: ORX), castrated rats treated for three months with PTH (1–84) 10 mg/kg/d (ORX + PTH1) and 50 mg/kg/d (ORX + PTH2), beginning three months after surgery. Data are expressed as mean + SD of 10 animals/group. Statistical significance p < 0.05: (a) versus SHAM; (b) versus ORX; and (c) versus ORX + PTH1.
Trabecular microarchitecture
Six months after surgery, most of the variables characterizing bone structure (from micro-CT) were significantly different in the ORX group with respect to the SHAM group. ORX rats exhibited a significant decrease in BV/TV in the distal femora (p < 0.05). This decrease in BV/TV was due to a significant increase in Tb.Sp and a decrease in Tb.N. The high dose of PTH totally prevented the changes occurred in ORX, showing similar values of BV/TV, Tb.Sp and Tb.N to those obtained in the SHAM group. Although the low dose of PTH restored BV/TV values, there were no significant changes in Tb.Sp and Tb.N. However, a tendency to reach SHAM values was observed ().
Figure 2. Femoral trabecular analysis by microcomputed tomography: bone volume/total volume (BV/TV), trabecular number (Tb.N), trabecular separation (TbS), trabecular pattern factor (Tb.Pf), structure model index (SMI) and degree of anisotropy (DA). Six-month-old male Wistar rats at the beginning of the study, distributed into four groups: Sham-operated rats (SHAM), castrated rats (orchidectomy: ORX), castrated rats treated for three months with PTH 10 mg/kg/d (ORX + PTH1) and PTH 50 mg/kg/d (ORX + PTH2), beginning three months after surgery. Data are expressed as mean + SD of 10 animals/group. Statistical significance p < 0.05: (a) versus SHAM; (b) versus ORX; and (c) versus ORX + PTH1.
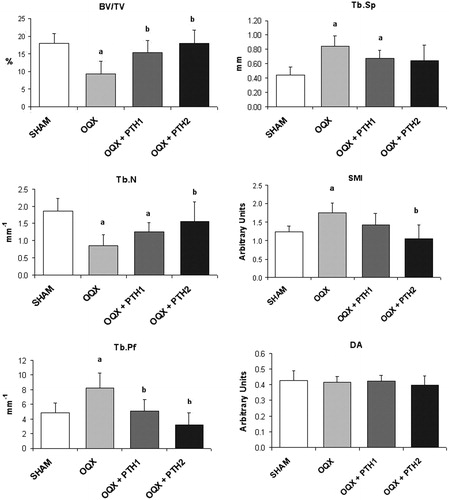
Orchidectomy also induced significant changes in non-metric parameters. SMI was significantly increased due to surgery, indicating a transformation of trabecular bone from plate- to rod-like structure. This effect observed in ORX rats was reversed with PTH treatment. Tb.Pf also increased in the ORX group, indicating a low connectedness of trabeculae. In comparison with the ORX group, treated rats showed a significant decrease in this parameter. The DA did not reflect any change between the ORX and SHAM groups nor between treated rats with respect to SHAM and ORX groups ().
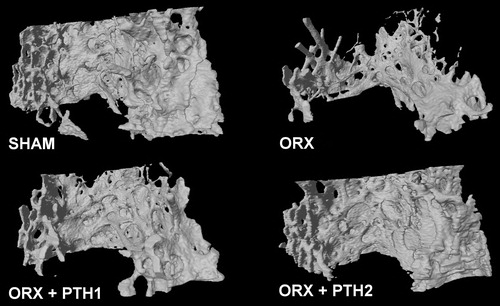
shows a representative image of bone trabecular microarchitecture in all the groups studied.
Figure 3. Representative images of bone trabecular 3D microarchitecture in distal femur obtained by microcomputed tomography. Study formed by sham-operated rats (SHAM), castrated rats (orchidectomy: ORX) and castrated rats treated for three months with PTH 10 mg/kg/d (ORX + PTH1) and PTH 50 mg/kg/d (ORX + PTH2).
Cortical microarchitecture
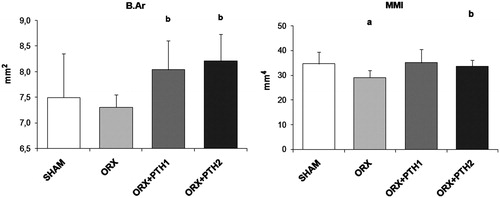
Orchidectomy produced a significant decrease in MMI with respect to SHAM group. This variable was restored after treatment with both doses of PTH. Orchidectomy did not produce any change in B.Ar in comparison to SHAM rats. However, both treatments induced a significant increase in this parameter with respect to the SHAM group ().
Figure 4. Cortical microarchitecture analysis: mean total crossectional bone area (B.Ar) and mean polar moment of inertia (MMI). Six-month-old male Wistar rats at the beginning of the study, distributed into four groups: Sham-operated rats (SHAM), castrated rats (orchidectomy: ORX), castrated rats treated for three months with PTH 10 mg/kg/d (ORX + PTH1) and PTH 50 mg/kg/d (ORX + PTH2), beginning three months after surgery. Data are expressed as mean + SD of 10 animals/group. Statistical significance p < 0.05: (a) versus SHAM; (b) versus ORX; (c) versus ORX + PTH1.
Biomechanical properties
Animals that underwent orchidectomy exhibited significantly lower levels of the extrinsic properties, including ultimate force, stiffness and work to failure. The effect of orchidectomy reverted with both doses of PTH. However, the values of stiffness were significantly higher in the ORX + PTH2 group, than in the ORX + PTH1 group ().
Table 1. Biomechanical properties of femora determined by three-point bending assessment.
Despite the fact that intrinsic properties were not affected by orchidectomy, when compared to the SHAM rats, the dose of 50 µg/Kg of PTH (1–84) produced a significant improvement in young modulus and ultimate stress, as compared with all other groups ().
Bone remodeling biochemical markers
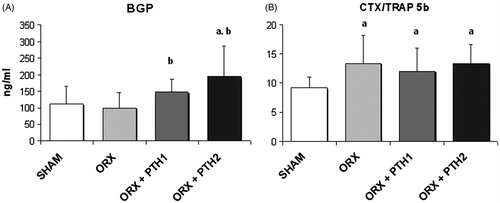
shows levels of biochemical markers of bone turnover. Six months after orchidectomy, untreated ORX rats were observed as having the same levels of BGP with respect to the SHAM rats. Treatment with both doses of PTH produced a significant increase in BGP levels (ORX + PTH1, p < 0.05; ORX + PTH2, p < 0.01) as compared with the ORX group, and the BGP values of the ORX + PTH1 group were similar to those of the SHAM group. Nevertheless, levels of BGP of ORX + PTH2 group were significantly higher than those of the SHAM group. The resorption index CTX/TRAP 5 b increased significantly in the untreated ORX group and the same levels maintained in both treated groups (ORX, p < 0.05; ORX + PTH1, p < 0.05; ORX + PTH2, p < 0.05).
Figure 5. Biochemical markers of bone turnover. (A) BGP, serum bone Gla-protein (ng/ml) and (B) CTX/TRAP5b index, serum C-telopeptide of type I collagen (ng/ml)/5b isoenzyme of tartrate resistant acid phosphatase (U/L). Six-month-old male Wistar rats at the beginning of the study, distributed into four groups: Sham-operated rats (SHAM), castrated rats (orchidectomy: ORX), castrated rats treated for three months with PTH 10 mg/kg/d (ORX + PTH1) and PTH 50 mg/kg/d (ORX + PTH2), beginning three months after surgery. Data are expressed as mean + SD of 10 animals/group. Statistical significance p < 0.05: (a) versus SHAM; and (b) versus ORX.
Discussion
Male osteoporosis is emerging as a central theme in bone research. It is firmly established that androgen withdrawal, induced by gonadectomy, results in decreased bone mass in adult rodents [Citation25,Citation26]. Like most animal models of low bone mass, the rat develops no fragility fractures, although mechanical testing of rat bones substitute as a predictor of bone fragility.
Different effects of androgens and estrogens are seen with gonadectomy when comparing the effects of orchidectomy in male (ORX) versus ovariectomy in the female (OVX) rats. OVX in the female results in decreased trabecular area in the metaphysis with increased osteoclast number, suggesting that estrogen protects trabecular bone predominantly through inhibition of osteoclast recruitment and activity. In the male, ORX produces a decrease in trabecular area in the metaphysis with increase of osteoclast number, resulting in trabecular osteopenia in the secondary spongiosa and in contrast with the female, the cortical bone periosteal formation was reduced [Citation27].
Tezval et al. [Citation28] studied the effect of PTH (1–34) 4 μg/kg daily for 5 weeks in sexually matured male Sprague-Dawley rats, 12 weeks after orchidectomy, and Montero et al. [Citation27] also treated adult male Wistar rats for 10 weeks with daily injections of rat PTH (1–34) 4 μg/kg/d, 24 weeks after orchidectomy. In both cases, PTH (1–34) was able to treat the hypogonadism-induced bone loss of trochanteric region of the rat femur, but there is no evidence that in vivo or clinical studies have been performed to reveal the effect of PTH (1–84) in trabecular or cortical bone, under a situation of androgenic depletion. However, PTH (1–84) has already been used in a model of OVX rats. After 12 months of treatment, a significant increase in LBMD was observed with respect to untreated OVX animals. On the other hand, no differences were found with respect to the effects produced by PTH (1–34) and PTH (1–84) on OVX rats [Citation29].
To determine the extent to which both PTH peptides will be equally capable to reverse the effect of loss of androgens in this model of male osteoporosis, we designed the study using our experience obtained from previous widely validated experimental models [Citation30]. While establishing study design of studies using rat models of osteoporosis, it is necessary to take into consideration three important features: the age of the animals at the moment of gonadectomy, the expected potency of the drug and the dose to be used, the duration of the treatment and the duration of the bone depletion period. Regarding the age of the animals at the moment of gonadectomy, most studies have used young animals (<6 months old) with a high rate of bone modeling. Thus, sexually matured ORX rats represent a better model for assessing the effects of agents intended to treat human male bone loss [Citation31]. Analyzing several studies performed with PTH (1–84) in OVX rats, different dose ranges have been used: from 25 μg/kg/d on 3 d/week to 100 μg/kg/d, 5 d/week. Proper choice of dose seems to be critical to the possible translation of the results to the clinical practice, as it is already known that a relationship between dose and risk of bone malignancies has been described for experimental use of bone anabolic treatments. As this is the first study performed in this model with PTH (1–84), we were aimed to use two doses: 10 and 50 μg/kg, which correspond on a molar basis to about 5 and 25 μg/kg of PTH (1–34), as used in most of the studies reviewed. Moreover, the lower dose of 10 μg/kg results in a PTH exposure that is closer to the clinical 100 μg dose used in humans (about 4–5 times greater) and was selected for assessing the carcinogenic potential in the skeleton, while used for 2 years in 9–11-month-old Fisher 344 male rats, where bone mass significantly increased [Citation32]. Interestingly, this dose was estimated to provide a systemic exposure to PTH that was 4.6-fold greater than the systemic exposure in humans following a 100 μg/d dose, which affords a wide safety margin following the clinically recommended dose for humans [Citation33]. We also used a higher dose in our study (50 μg/kg/d) as it was a study of a limited duration of treatment with PTH (1–84) (3 months), and we assumed the safety profile to be appropriate and would allow us to perform comparisons in efficacy with the doses used in previous studies with PTH (1–34).
We started the treatment three months after orchidectomy in order to achieve a decrease in BMD that could be measured by techniques of DEXA and to reproduce the clinical features of osteoporosis [Citation34]. After orchidectomy, we found low bone mass (loss of 11% of femoral bone by DEXA). Similar to other authors [Citation35], our findings show that lack of androgens in ORX Wistar rats led to a significant decrease in both femoral and LBMD three months after surgery. This loss of bone partially reversed after the administration of 10 μg/kg/d of PTH (1–84) and was completely recovered with the higher dose of 50 μg/kg/d. Moreover, peripheral quantitative computed tomography and biomechanical testing were used to explain the effects of PTH treatment in the trabecular and cortical bone envelopes, at the distal femur, a skeletal site that contains substantial amounts of both types of trabecular and cortical bone. Bone microarchitecture values were concordant with DEXA as the high dose of PTH (1–84) totally restored the changes occurred in ORX, showing similar values of BV/TV, Tb.Sp and Tb.N to those obtained in SHAM group. The low dose of PTH also restored BV/TV values with a tendency toward SHAM values in Tb.Sp and Tb.N. Audran et al. [Citation36] studied the relationship between the microarchitecture values obtained in the proximal region of the tibia of ORX Wistar rats and the histomorphometric values in transiliac bone biopsies of men with lumbar osteopenia due to glucocorticosteroid-induced and primary osteoporosis. Their results strongly suggest that bone trabecular microarchitecture is a major and independent determinant of vertebral fracture in men with osteoporosis. Thus, the reversibility of the deteriorate histomorphometric values while using PTH (1–84) should lead to the design of further studies in men in order to find out how PTH (1–84) could also prevent osteoporotic fractures.
As previously described [Citation37], micro-CT examination of cortical bone revealed a mild, but statistically significant effect of castration on cortical bone, mainly decreasing the polar moment of inertia, which reflects the ability of bone to resist torsion. Due to the anabolic effect of PTH on bone, both doses of PTH avoided the decrease in cortical parameters produced by orchidectomy, including cortical bone area and polar moment of inertia.
With respect to biomechanical properties, it is important to distinguish between extrinsic or structural properties and intrinsic or material properties. When a force (load) is applied, bone suffers a deformation (displacement). Extrinsic properties come from the load-displacement curve. Then, this curve is transformed into a stress–strain curve to obtain the intrinsic properties [Citation38]. In this work, we found that biomechanical properties were consistent with all other results. The clear decrease in the quality of the bone trabecular microstructure (decrease in BV/TV and Tb.N; increase in Tb.Pf and Tb.Sp) together with a light deterioration of the cortical microstructure leads to a decrease in bone strength specially reflected in the extrinsic or structural biochemical properties. Extrinsic biomechanical properties decreased significantly in the ORX group. The effect of orchidectomy was reversed with both doses of PTH (1–84). However, the value of stiffness obtained was significantly higher in the group treated with 50 μg/kg/d than in the group treated with 10 μg/kg/d. On the other hand, although intrinsic properties were not affected by orchidectomy, the dose of 50 µg/Kg produced a significant improvement in young modulus and ultimate stress, as compared with all other groups.
In accordance to Rissanen et al., in this work, we have used the CTX/TRAP resorption index. TRAP is a reliable marker of osteoclast number, and CTX correlates with osteoclast activity. This resorption index is particularly useful in situations where osteoclast activity is increased and osteoclast number is decreased, such as in the rat model of OVX [Citation39]. In this work, we have found that this resorption index was higher in ORX rats than in the SHAM rats. We also measured the bone formation marker BGP. We did not find significant differences in BGP levels between the ORX and SHAM groups. Other authors have described that bone turnover is probably only temporarily increased in rats which have undergone orchidectomy [Citation40]. Rats treated with PTH (1–84) (both doses) did not show any changes in the resorption index CTX/TRAP5b with respect to the ORX rats. Both doses of PTH (1–84) produced a significant increase in BGP levels with respect to the ORX animals, being in the case of the higher dose, even significantly higher than in the SHAM group. These results demonstrate the anabolic effect of PTH (1–84) and correlate with the results of other authors [Citation8,Citation41].
In conclusion, this work provides new information about the effects of PTH (1–84) on bone in an animal model of male osteoporosis. Although there are evident limitations when extrapolating these results to humans, we think that this model could establish the pharmacological bases to study the effect of PTH (1–84) on the osteoporosis in men. It is necessary to carry out clinical trials to evaluate these effects. Due to the fact that teriparatide is the current anabolic treatment for male osteoporosis, it would be worthwhile to perform comparative studies between this drug and PTH (1–84), analyzing their efficacy and safety.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This work was supported by a grant from Nycomed Pharma (Spain). Marta Martín-Fernández was a fellow of the Conchita Rábago Foundation.
References
- Dawson-Hughes B, Looker AC, Tosteson ANA, et al. The potential impact of new National Osteoporosis Foundation guidance on treatment patterns. Osteoporosis Int 2010;21:41–52
- Kamel HK. Male osteoporosis: new trends in diagnosis and therapy. Drugs Aging 2005;22:741–8
- Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 2002;23:279–302
- Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med 2007;357:905–16
- Kurland ES, Cosman F, McMahon DJ, et al. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 2000;85:3069–76
- Miyakoshi N. Effects of parathyroid hormone on cancellous bone mass and structure in osteoporosis. Curr Pharm Des 2004;10:2615–27
- Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434–41
- Greenspan SL, Bone HG, Ettinger MP, et al. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med 2007;146:326–39
- Hock JM. Anabolic actions of PTH in the skeletons of animals. J Musculoskelet Neuronal Interact 2001;2:33–47
- Poole KES, Reeve J. Parathyroid hormone – a bone anabolic and catabolic agent. Curr Opin Pharmacol 2005;5:612–17
- Hock JM, Gera I, Fonseca J, Raisz LG. Human parathyroid hormone-(1–34) increases bone mass in ovariectomized and orchidectomized rats. Endocrinology 1988;122:2899–904
- Hori M, Uzawa T, Morita K, et al. Effect of human parathyroid hormone (PTH(1–34)) on experimental osteopenia of rats induced by ovariectomy. Bone Miner 1988;3:193–9
- Stanislaus D, Devanarayan V, Hock JM. In vivo comparison of activated protein-1 gene activation in response to human parathyroid hormone (hPTH)(1–34) and hPTH(1–84) in the distal femur metaphyses of young mice. Bone 2000;27:819–26
- Kimmel DB, Bozzato RP, Kronis KA, et al. The effect of recombinant human (1–84) or synthetic human (1–34) parathyroid hormone on the skeleton of adult osteopenic ovariectomized rats. Endocrinology 1993;132:1577–84
- Ejersted C, Andreassen TT, Oxlund H, et al. Human parathyroid hormone (1–34) and (1–84) increase the mechanical strength and thickness of cortical bone in rats. J Bone Miner Res 1993;8:1097–101
- Borchers RE, Gibson LJ, Burchardt H, Hayes WC. Effects of selected thermal variables on the mechanical properties of trabecular bone. Biomaterials 1995;16:545–51
- Gala Paniagua J, Díaz-Curiel M, De la Piedra Gordo C, et al. Bone mass assessment in rats by dual energy X-ray absorptiometry. Br J Radiol 1998;71:754–8
- Feldkamp L, Davis L, Kress J. Practical cone-beam algorithms. J Opt Soc Am A 1984;6:612–19
- Hildebrand T, Ruegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc 1997;185:67–75
- Ulrich D, Van Rietbergen B, Laib A, Rüegsegger P. The ability of three-dimensional structural indices to reflect mechanical aspects of trabecular bone. Bone 1999;25:55–60
- Hildebrand T, Rüegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin 1997;1:15–23
- Harrigan TP, Mann RW. Characterization of microstructural anisotropy in orthotropic materials using a second rank tensor. J Mater Sci 1984;19:761–7
- Hahn M, Vogel M, Pompesius-Kempa M, Delling G. Trabecular bone pattern factor – a new parameter for simple quantification of bone microarchitecture. Bone 1992;13:327–30
- Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone 1993;14:595–608
- Wink CS, Felts WJ. Effects of castration on the bone structure of male rats: a model of osteoporosis. Calcif Tissue Int 1980;32:77–82
- Vanderschueren D, Van Herck E, Schot P, et al. The aged male rat as a model for human osteoporosis: evaluation by nondestructive measurements and biomechanical testing. Calcif Tissue Int 1993;53:342–7
- Montero M, Serfati D, Luna S, et al. The effectiveness of intermittent rat parathyroid hormone (1–34) treatment on low bone mass due to oestrogen or androgen depletion in skeletally mature rats. Aging Male 2010;13:59–73
- Tezval M, Serferaz G, Rack T, et al. Effect of parathyroid hormone on hypogonadism induced bone loss of proximal femur of orchiectomized rat. World J Urol 2011;29:529–34
- Fox J, Miller MA, Newman MK, et al. Daily treatment of aged ovariectomized rats with human parathyroid hormone (1–84) for 12 months reverses bone loss and enhances trabecular and cortical bone strength. Calcif Tissue Int 2006;79:262–72
- Curiel MD, Calero JA, Guerrero R, et al. Effects of LY-117018 HCl on bone remodeling and mineral density in the oophorectomized rat. Am J Obstet Gynecol 1998;178:320–5
- Kimmel DB. Animal models in osteoporosis research. In: Bilezekian J, Raisz L, Rodan G, eds. Principles of bone biology. San Diego, CA: Academic; 2002:1635–55
- Jolette J, Wilker CE, Smith SY, et al. Defining a noncarcinogenic dose of recombinant human parathyroid hormone 1–84 in a 2-year study in Fischer 344 rats. Toxicol Pathol 2006;34:929–40
- Vahle JL, Long GG, Sandusky G, et al. Bone neoplasms in F344 rats given teriparatide [rhPTH(1–34)] are dependent on duration of treatment and dose. Toxicol Pathol 2004;32:426–38
- Francisco JI, Yu Y, Oliver RA, Walsh WR. Relationship between age, skeletal site, and time post-ovariectomy on bone mineral and trabecular microarchitecture in rats. J Orthop Res 2011;29:189–96
- Hernandes L, Ramos AL, Micheletti KR, et al. Densitometry, radiography, and histological assessment of collagen as methods to evaluate femoral bones in an experimental model of osteoporosis. Osteoporosis Int 2012;23:467–73
- Audran M, Chappard D, Legrand E, et al. Bone microarchitecture and bone fragility in men: DXA and histomorphometry in humans and in the orchidectomized rat model. Calcif Tissue Int 2001;69:214–17
- Bagi CM, Hanson N, Andresen C, et al. The use of micro-CT to evaluate cortical bone geometry and strength in nude rats: correlation with mechanical testing, pQCT and DXA. Bone 2006;38:136–44
- De La Piedra C, Quiroga I, Montero M, et al. Daily or monthly ibandronate prevents or restores deteriorations of bone mass, architecture, biomechanical properties and markers of bone turnover in androgen-deficient aged rats. Aging Male 2011;14:220–30
- Rissanen JP, Suominen MI, Peng Z, Halleen JM. Secreted tartrate-resistant acid phosphatase 5b is a Marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif Tissue Int 2008;82:108–15
- Vanderschueren D, Van Herck E, Suiker AM, et al. Bone and mineral metabolism in aged male rats: short and long term effects of androgen deficiency. Endocrinology 1992;130:2906–16
- Hodsman AB, Fraher LJ, Ostbye T, et al. An evaluation of several biochemical markers for bone formation and resorption in a protocol utilizing cyclical parathyroid hormone and calcitonin therapy for osteoporosis. J Clin Invest 1993;91:1138–48
