Abstract
Aim: The aim of this study was to investigate the effect of pravastatin treatment on diminished corpus cavernosum (CC) function associated with aging.
Methods: Male rats were divided into three groups as adult rats (12–14 weeks old), aged rats (72–80 weeks old) and aged rats given 10 mg/kg/d pravastatin in drinking water for six weeks. Blood pressure was measured by tail-cuff method. Total cholesterol, low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, triglycerides and testosterone levels were estimated in blood. Changes in expression levels of endothelial nitric oxide synthase (eNOS), phosphorylated eNOS (p-eNOS) (Ser-1177), neuronal nitric oxide synthase (nNOS), NADPH oxidase subunit gp91phox, Rho A and Rho kinase (ROCK2) in CC were assessed by immunohistochemistry. Nitric oxide (NO)-mediated endothelium-dependent and neurogenic CC relaxation were evaluated by acetylcholine (ACh, 0.1 nM–100 µM) and electrical field stimulation (EFS; 30 V, 5 ms, 2–32 Hz), respectively.
Results: In aged rats, NO-mediated, both endothelium-dependent and neurogenic CC relaxation, were significantly impaired as compared to adult rats. Besides, eNOS, p-eNOS and nNOS expressions decreased significantly in CC from aged rats, while gp91phox, RhoA and ROCK2 expressions increased significantly. The diminished relaxation in response to ACh or EFS as well as the changes in expression of these proteins in aged rats were significantly improved by pravastatin treatment.
Conclusion: Pravastatin improves NO-mediated CC relaxations of aged rats probably by inhibiting NADPH oxidase/Rho kinase pathways, and this effect does not seem to be associated with lipid lowering effect of this drug.
Introduction
Erectile dysfunction (ED), which has been defined as the permanent inability to achieve or maintain an erection sufficient rigidity of the penis to allow satisfactory sexual intercourse, is a significant problem among aging men [Citation1,Citation2]. The prevalence of ED was higher in the older age groups, peaking in men aged 70 years and older (64%) [Citation3]. As life expectancy increases, men are seeking to protect their sexuality into old age. Age-related ED has been postulated to be caused by an alteration in a precise balance between endothelium-derived contractile and relaxant factors, which is characterized by decreased nitric oxide (NO)-mediated neurogenic and endothelium-dependent relaxation of corpus cavernosum (CC) [Citation4–6], and upregulation of the RhoA/Rho kinase contractile pathway [Citation7,Citation8] in the penis. Thus, it is expected that agents interfering with such pathways may offer additional options for treating ED in aging.
Previous studies suggest that the clinical benefits of hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) may, in part, be attributed to mechanisms independent of their cholesterol-lowering effects, those benefits being explained by the “pleiotropic” effects of statins [Citation9]. In a previous preclinical study, rosuvastatin treatment has been shown to correct defective NO-mediated nerve and vascular function in diabetic mice independent of cholesterol lowering effects [Citation10]. On the other hand, clinical studies have produced conflicting data in this area. Results from a recent study have indicated that atorvastatin alone seems to improve erectile function compared to not using any medication [Citation11], whereas findings from other clinical studies have not supported the use of simvastatin as erectogenic medication [Citation12,Citation13]. Statins previously shown to have an antioxidative effect [Citation14,Citation15] are promising therapeutic alternative to influence some aspects of vascular aging. Besides contributing to the regulation of lipid profile, these drugs may increase NO bioavailability by enhancing both endothelial nitric oxide synthase (eNOS) and neuronal nitric oxide synthase (nNOS) expression [Citation16,Citation17]. Moreover, statins inhibit the synthesis of isoprenoid intermediates of the cholesterol synthesis pathway, such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate (GGPP), which serve as important lipid attachments for the post-translational modification of a variety of proteins, including the small GTPases such as RhoA [Citation18,Citation19]. Although the recent evidence suggests that RhoA/Rho kinase pathway may play an important role in ED in aging, the detailed effects of statins on CC dysfunction related with aging remain to be fully elucidated. It is also not known whether statins influence the neuronal NO system in parallel to an action on endothelium of CC, which could have important implications for age-related impotence.
This study was designed to test the hypothesis that pravastatin, an inhibitor of HMG-CoA reductase that belongs to lipid-lowering drugs, is able to improve age-related dysfunction in the rat CC without affecting plasma lipid levels and to elucidate the role(s) of NADPH oxidase/Rho kinase/NO pathway its favorable effect on erectile responses. We have also evaluated whether pravastatin treatment could interfere with NOS, a critical molecular member in NO-cGMP pathway, and thereby potentiate the effect of by phosphodiesterase type 5 (PDE-5) inhibitors in rat CC.
Materials and methods
Experimental procedures
All animal experiments were carried out with the approval of the Animal Ethics Committee of Akdeniz University Medical Faculty, Antalya, Turkey. Briefly, adult (12–14 weeks, n = 8) and aged (72–80 weeks, n = 16) male Wistar rats were used. Aged rats were randomly divided into two groups (n = 8 in each group): the first group in which pravastatin (10 mg/kg/d body weight) was given to rats in their drinking water for six weeks and the second group receiving only tap water. This dosage and method of pravastatin administration were chosen according to previous reports showing that pravastatin at a dose of 10 mg/kg did not alter blood lipid levels [Citation20,Citation21]. Moreover, the previous experiments showed that the endothelium-dependent relaxation was restored by this dose of pravastatin without lowering plasma cholesterol [Citation22]. Thus, we used this dose of pravastatin in this experiment. All rats had free access to food and water in an animal room that was maintained at 22 °C ± 0.5 °C with a 12 h light–dark cycle. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by tail-cuff method with a pressure meter (MP150 system, Commat Ltd., Ankara, Turkey).
Blood biochemical assays
All rats were weighted and then anesthetized with a cocktail of ketamine hydrochloride (90 mg/kg) and xylazine (10 mg/kg) administered intramuscularly before sacrifice of each rat, and blood samples that were obtained from the abdominal vein were collected into tubes. Thereafter, serum was separated by centrifugation at 4000 × g for 10 min at 4 °C. Total cholesterol, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, triglycerides and testosterone levels were measured (using commercial kits from Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s specifications.
Isolated organ bath technique
The rats were humanly killed by cervical dislocation. Penises were surgically removed at the level of the crural attachments to the puboischial bones and promptly placed in a Petri dish containing Krebs solution (containing in mM: NaCl, 118; KCl, 5; NaHCO3, 25; KH2PO4, 1.0; MgSO4, 1.2; CaCl2, 2.5 and glucose, 11.2), bubbled with a mixture of 95% O2 and 5% CO2. The glans penis and urethra were excised, and the CC tissue was then dissected free from the tunica albuginea. The CC was separated by cutting the fibrous septum between them. Two cavernosal strips of approximately equal size (2 × 2 × 8 mm) were obtained from each penis. Each cavernosal strip was tied with silk in one organ chamber with one end fixed to a tissue holder and the other secured to a force transducer. Cavernosal strips were maintained in the organ baths with filled with Krebs solution maintained at 37 °C and gassed with 95% O2 and 5% CO2 to obtain a pH of 7.4. Isometric tension was continuously measured using an isometric force transducer (FDT-5 A, Commat Ltd.), connected to a computer-based data acquisition system (MP36, Commat Ltd.). The resting tension was adjusted to 0.5 g, which is optimal for inducing the maximal contraction and allowed to equilibrate for 60 min. This optimal resting tension was calculated as follows: the strips were stretched over a range of resting tension from 0.2 to 1.5 g; and after equilibration for 60 min, the contractile responses to phenylephrine (Phe; 10 μM) were measured. During the resting periods, organ bath solution was changed every 15 min. For the relaxation studies, CC strips were precontracted with Phe (10 μM). This concentration was determined from the cumulative contraction-response curves to achieve 80% of the maximum contraction.
Protocol
In the first set of experiments, CC tissues were challenged with Phe (10 μM). When the Phe-induced contraction reached a plateau level, relaxation responses were examined using an endothelium-specific vasodilator, acetylcholine (ACh, 0.1 nM–100 μM), by addition of increasing concentrations of agonist to the baths in a cumulative manner. The tissue response was allowed to reach a stable plateau (2–4 min) before each successive addition of the agonist. Then, the solution was replaced for the Krebs solution, and the tension was stabilized at baseline values with frequent renewal of the solution. After 60 min, CC strips were contracted with Phe (10 μM), and other concentration-effect curves were obtained with sodium nitroprusside (SNP; 10 nM–1 mM).
In a separate set of experiments, cavernosal strips were contracted with Phe (10 μM), and relaxation was evoked by electrical field stimulation (EFS). Electrical stimuli were applied to strips placed between platinum pin electrodes, which were connected to a May STPT 03 Research Stimulator (Commat Ltd.). EFS was conducted at 30 V, 5 ms pulse width and trains of stimuli lasting 10 s at varying frequencies (2–32 Hz) for 2 min. These experiments were performed in the presence of adrenergic and muscarinic receptor blocking agents (10 µM guanethidine and 1 µM atropine, respectively) in the bathing medium to obtain nonadrenergic noncholinergic conditions.
Furthermore, in order to investigate whether long-term pravastatin treatment has a beneficial effect on sildenafil citrate-induced relaxation of CC, concentration-response curves for sildenafil citrate (0.1 nM–1 μM) were constructed in precontracted CC strips of all groups.
Tissue processing and immunohistochemistry
CC tissues obtained from adult rats, aged rats and aged rats treated with pravastatin were fixed in 10% formalin and were processed routinely for paraffin embedding. Paraffin-embedded tissue samples were cut into 5 μm thick sections and mounted on SuperFrost Plus slides (Erie Scientific Company, Portsmouth, NH). For eNOS, phosphorylated eNOS (p-eNOS), nNOS, gp91phox, RhoA and Rho kinase (ROCK2) immunostainings, sections were deparaffinized in xylene and rehydrated in a graded series of alcohol. For antigen retrieval, slides were placed in 10 mM citrate buffer (pH 6.0) and were microwaved twice for 5 min. Tissue sections were blocked for endogenous peroxidase activity with methanol containing 3% H2O2 for 10 min. After several washes with phosphate-buffered saline (PBS), to eliminate nonspecific binding, sections were incubated with Ultra V Block (Labvision, Freemonth, CA) for 7 min at room temperature. Rabbit eNOS (sc-654, Santa Cruz, CA), goat p-eNOS (Ser 1177) (sc-12972, Santa Cruz, CA), rabbit nNOS (Cell Signaling 4236, Beverley, MA), rabbit gp91phox (bs-3889, Bios, CA), rabbit RhoA (bs-1180, Bios, CA) and rabbit Rho kinase (ROCK2) (bs-1205, Bios, CA) primary antibodies were applied in a dilution of 1:100, 1:100, 1:50, 1:50, 1:50 and 1:50, respectively. Sections were washed in PBS and incubated with biotinylated horse anti-rabbit and anti-goat IgG (Vector, Burlingame, CA) secondary antibodies at 1:500 dilution for all antibodies for 1 h at room temperature. After several PBS rinses, the antigen–antibody complex was detected by using an avidin–biotin horseradish peroxidase complex with a Universal LSAB Kit (Dako, Glostrup, Denmark). Diaminobenzidine (3,3-diaminobenzidine tetrahydrochloride dihydrate; Sigma, St. Louis, MO) was used as the chromogen. Sections were mounted with Permount (Fisher Chemicals, Fair Lawn, NJ) on glass slides and then evaluated under light microscope. For controls, sections were incubated with rabbit and goat serum (Dako) at the same concentrations with the primary antibodies. Pictures were taken with Spot Advanced Imaging Software (Diagnostic Instruments, Inc., Sterling Heights, MI). ImageJ (NIH, Bethsada, MD) for microscopy software was used to quantify the extent of immunostainings.
Materials
ACh hydrochloride, Phe chloride, sildenafil citrate, SNP and the salts for the Krebs solution were purchased from Sigma Chemical. In this study, pravastatin was administered as the clinical formulation (Pravachol 10 mg, Deva, Istanbul, Turkey). All drugs were prepared fresh daily during experiments and were dissolved in distilled water before use, except in the case of sildenafil (initially dissolved in dimethyl sulfoxide).
Statistical analysis
All values are expressed as mean ± SEM. Responses to ACh and EFS are expressed as percentages of the reversal of the tension developed in response to Phe. Statistical analysis of the results were performed by one-way analysis of variance or Student’s t-test, as appropriate. Post hoc comparisons were done using Newman–Keuls multiple comparison test. A p value lower than 0.05 was considered significant.
Results
Body weights and biochemical analysis
Body weight was significantly increased in aged rats (). No significant difference was observed in the body weights of the rats between aged rats treated with or without pravastatin. SBP and DBP did not change with aging or pravastatin treatment (). Besides, plasma levels of total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides and testosterone were not affected by pravastatin at the dosage used ().
Table 1. Body weight, systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels in adult rats, aged rats and aged rats treated with pravastatin.
Table 2. Total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides and testosterone levels in adult rats, aged rats and aged rats treated with pravastatin.
Functional studies
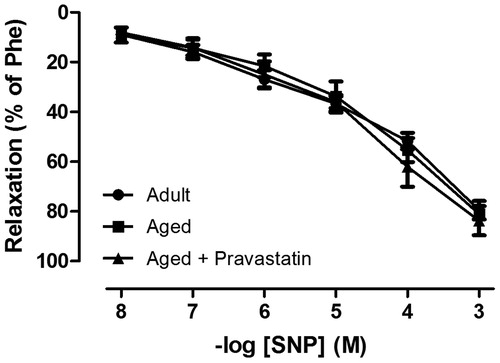
Age-related decline in male erectile function was evidenced by the significant decrease in relaxation response of CC to both electrical stimulation and ACh. As expected, both endothelium-dependent response to ACh and neurogenic response to EFS were significantly decreased in CC stips from aged versus adult rats showing age-related ED ( and ). Six weeks treatment with pravastatin improved endothelium-dependent relaxation to ACh in CC obtained from aged rats (). Moreover, this treatment caused a significant increase in relaxation response to EFS over the range of 2–32 Hz (). In contrast, neither aging nor aging plus pravastatin treatment affected SNP-induced relaxation of rat CC ().
Figure 1. The NO-mediated endothelium-dependent relaxation response of corpus cavernosum to acetylcholine (ACh, 0.1 nM–100 μM) in adult rats, aged rats and aged rats treated with pravastatin. All values are expressed as mean ± SEM. n = 8 for all groups.*p < 0.05 as compared with adult rats, #p < 0.05 as compared with aged rats.
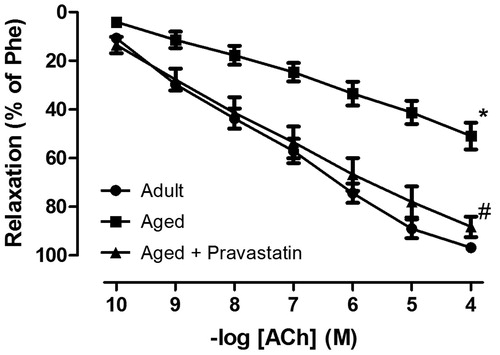
Figure 2. The NO-mediated neurogenic relaxation response of corpus cavernosum to electrical field stimulation (EFS, 2–32 Hz) in adult rats, aged rats and aged rats treated with pravastatin. These experiments were performed in the presence of adrenergic and muscarinic receptor blocking agents (10 µM guanethidine and 1 µM atropine, respectively). All values are expressed as mean ± SEM. n = 8 for all groups. *p < 0.05 as compared with adult rats, #p < 0.05 as compared with aged rats.
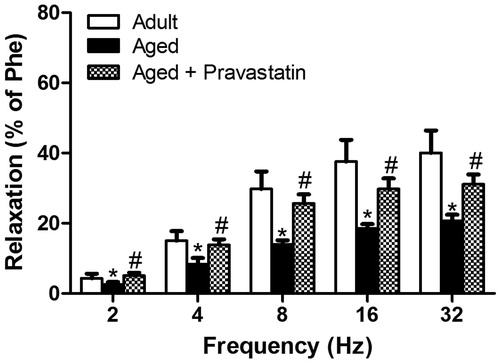
Figure 3. The relaxation response of corpus cavernosum to sodium nitroprusside (SNP, 10 nM–1 mM) in adult rats, aged rats and aged rats treated with pravastatin. All values are expressed as mean ± SEM. n = 8 for all groups.
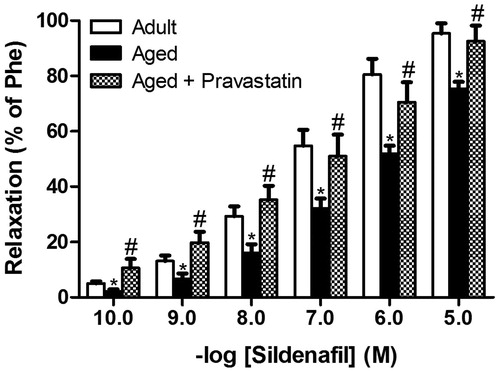
Cumulative addition of the PDE-5 inhibitor sildenafil (0.1 nM–1 μM) concentration-dependently relaxed rat CC (). The relaxant response induced by sildenafil was partially inhibited by aging. The diminished relaxation response of CC to sildenafil was significantly enhanced by pravastatin treatment in aged rats, as shown in . Treatment with pravastatin resulted in a significant increase in Emax value for sildenafil in aged rats (75.3 ± 2.6% for aged rats and 92.5 ± 5.7% for aged rats treated with pravastatin).
Figure 4. Relaxation response of corpus cavernosum induced by various concentration of sildenafil citrate (0.1 nM–1 μM) in adult rats, aged rats and aged rats treated with pravastatin. All values are expressed as mean ± SEM. n = 8 for all groups. *p < 0.05 as compared with adult rats, #p < 0.05 as compared with aged rats.
The expression levels of eNOS, p-eNOS, nNOS, gp91phox, RhoA and ROCK2 in corporal tissue
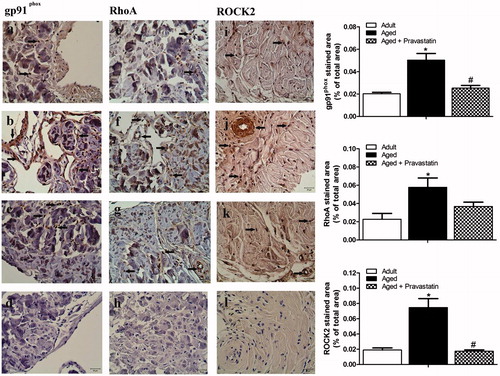
In order to elucidate the of cellular expression of eNOS, p-eNOS and nNOS, CC tissue sections were immunohistochemically stained with anti-eNOS, anti-p-eNOS and anti-nNOS antibodies, and results were presented in . eNOS, p-eNOS and nNOS expressions in CC tissue of adult rats were shown in Figures 5a, e and i, respectively. There was a significant decrease in eNOS, p-eNOS and nNOS protein expressions in the CC of aging rats when compared to adult rats (). Treatment with pravastatin of aged rats increased the expressions of eNOS, p-eNOS and nNOS (). The negative control immunostainings of eNOS, p-eNOS and nNOS were represented in Figures 5d, h and l. gp91phox, RhoA and Rho kinase (ROCK2) expressions in CC tissue of adult rats were shown in Figures 6a, e and i, respectively. On the other hand, gp91phox, RhoA and Rho kinase (ROCK2) expressions significantly enhanced in CC tissue of aged rats when compared to adults (). However, the intensity of gp91phox, RhoA and ROCK2 expressions in corporal tissue of aged rats treated with pravastatin decreased when compared to aged rats without pravastatin treatment (). The negative control immunostainings of gp91phox, RhoA and ROCK2 were represented in Figures 6d, h and l.
Figure 5. Representative photomicrographs for eNOS (a–d), p-eNOS (e–h) and nNOS (i–l) immunohistochemistry in adult (a, e and i), aged (b, f and j) and aged treated with pravastatin (c, g and k) corpus cavernosum tissues. Insets in d, h and l represent the negative control immunostainings of eNOS, p-eNOS and nNOS, respectively. Arrows indicate eNOS, p-eNOS and nNOS expressions in corpus cavernosum tissues. The graphs show image analysis results after immunohistochemistry. *p < 0.05 as compared with adult rats and #p < 0.05 as compared with aged rats.
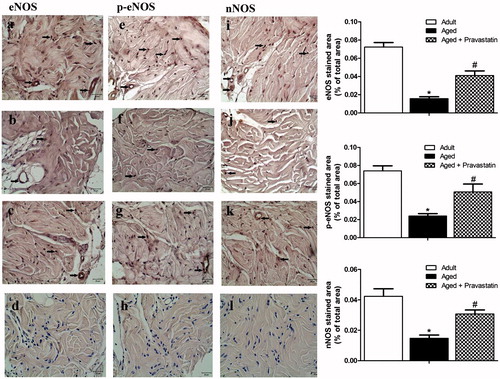
Figure 6. Representative photomicrographs for gp91phox (a–d), RhoA (e–h) and ROCK2 (i–l) immunohistochemistry in adult (a, e and i), aged (b, f and j) and aged treated with pravastatin (c, g and k) corpus cavernosum tissues. Insets in d, h and l represent the negative control immunostainings of gp91phox, RhoA and ROCK2, respectively. Arrows indicate gp91phox, RhoA and ROCK2 expressions in corpus cavernosum tissues. The graphs show image analysis results after immunohistochemistry. *p < 0.05 as compared with adult rats and #p < 0.05 as compared with aged rats.
Discussion
This study is the first to describe a restorative effect of pravastatin treatment on age-related dysfunction of rat CC without affecting lipid profile, suggesting a novel role for statins in protecting against age-related ED. Our findings also provide the first evidence that the mechanism(s) underlying the enhanced NO-mediated relaxation of CC in aged rats treated with pravastatin seem to be related with an increase of eNOS, p-eNOS and nNOS expression, and decreased expression of the gp91phox subunits of NADPH oxidase and Rho kinase.
It is widely known that both nNOS and eNOS play an important role in the mechanism of erectile function. After sexual stimulation, NO is synthesized by nNOS and eNOS and released from nerve endings and endothelium in the penis. Neurogenic NO is still considered the most important factor for immediate relaxation of CC [Citation23,Citation24]. However, endothelially generated NO can facilitate attainment and maintenance of a full erection. Conditions associated with reduced function of nerves and endothelium can result in arterial insufficiency and defective CC relaxation. Among them, physiological aging is a significant risk factor in the on-set of male ED, and an imbalance in factors that modulate cavernosal smooth muscle tone may play a role in these altered penile hemodynamic mechanisms [Citation25]. Accordingly, the results of this study showed that aging resulted in a decline in erectile function, as indicated by a decrease in both endothelium-dependent and neurogenic CC relaxation of aged rats. This result confirmed previous findings indicating that aging was able to induce ED in experimental and clinical studies [Citation2,Citation5,Citation25]. However, the impairment in the nitrergic system may not associated with the sensitivity of the CC to NO since the relaxation produced by SNP, a NO donor, was not changed by physiological aging. This detrimental effect of aging on CC function is not related with an increase in arterial blood pressure because, in our experimental conditions, SBP and DBP were not significantly affected as a consequence of aging. Previous findings suggested that NOS isoforms vary with aging and their changes affect erectile function [Citation4,Citation26–28]. It is well known that impaired endothelium-dependent relaxation of CC in aged rats might be due to changes in eNOS expression and/or activity. The catalytic activity of eNOS toward NO generation is increased when phosphorylated at Ser 1177 by numerous protein kinases [Citation29–31]. Results of this study showed that both total eNOS protein expression and eNOS phosphorylation at Ser 1177 were decreased in CC of aged rats compared with that of control rats. Our results also showed that expression of nNOS in rat CC significantly reduced by aging, resulting in defective NO-mediated neurogenic CC relaxation. Based on the explanations above, it is possible to suggest that there may be a decrease in NO generation under the influence of aging.
Recent findings suggested an important role for RhoA/Rho kinase pathway in the regulation of cavernosal smooth muscle tone and that changes in this pathway may contribute to ED in various patient subgroups. RhoA is a member of the Ras GTPase family and a key intracellular regulator of cellular responses including migration and contraction of smooth muscle [Citation32]. Rho kinase, one of the downstream targets of RhoA, has been shown to be involved in erectile function [Citation33]. Accordingly, our results also showed that expressions of RhoA and Rho kinase (ROCK2) proteins were increased in CC tissue of aged rats. There is an interesting inverse functional relationship between the NO/cGMP and RhoA/Rho kinase signaling pathways within the vasculature [Citation34]. Previous studies indicated that upregulation of RhoA/Rho kinase suppresses eNOS and nNOS expression in the penis [Citation8,Citation35]. By this route, RhoA/Rho kinase pathway activation in CC may cause functional impairment of erectile function at the cellular level and reduced ability of CC tissue to produce NO when stimulated by ACh, an endothelium-dependent relaxant agent, or by EFS. Indeed, we found increased protein expression of RhoA and ROCK2 in CC of aged rats, so upregulation of RhoA/Rho kinase signaling is suspected of being involved in the development of age-related CC dysfunction and is inversely related to NO production in CC tissue. ED in aged rats is associated with decreased NO bioavailability in erectile tissue due to upregulation of NADPH oxidase subunit gp91phox and downregulation of nNOS/p-eNOS [Citation36]. In accordance with the earlier findings, results of this study demonstrated an increase in the expression of gp91phoxin CC of aged rats. In line with these explanations, it is possible to suggest that impaired neurogenic or endothelium-mediated CC relaxation of aged rats could be prevented by inhibition of overactivated NADPH oxidase/Rho kinase signaling, thus improving eNOS and nNOS activity.
It is widely admitted that intermolecular differences between the different HMG-CoA inhibitors can contribute to distinct pharmacologic and pleiotropic actions [Citation37]. Previous studies showed all statins were effective in partially reversing endothelial dysfunction caused by hypercholesterolemia [Citation38,Citation39]. Those studies reported that the effect was similar in the different drugs, except for pravastatin, whose effect on endothelium-dependent relaxation was more significant. Furthermore, it has been shown that pravastatin, but not atorvastatin, partially restored ACh-induced pulmonary artery vasodilation in monocrotaline-induced pulmonary hypertension in rats due to its pleiotropic effects [Citation40]. These authors also demonstrated that cholesterol lowering was not involved in the protective effect of pravastatin against monocrotaline-induced pulmonary hypertension [Citation41]. On the other hand, accumulating evidence indicates that one important mechanism of ED is the excessive apoptosis of erectile tissues, which can be caused by a lot of risk factors of ED and thereby reduce penile erectile function [Citation42]. It is known that lovastatin, simvastatin, atorvastatin, fluvastatin and cerivastatin, which are hydrophobic statins, markedly reduced cell viability via apoptotic cell death [Citation43]. Pravastatin, which is a hydrophilic statin, however, did not induce endothelial cell apoptosis. Based on the results presented above, among the various statin drugs, we decided to evaluate the effect of long-term pravastatin treatment on erectile functions in aging rats, without altering the lipid profile. This investigation revealed for the first time that pravastatin treatment provides protection against aged-induced CC dysfunction, as indicated by an improvement in both neurogenic and endothelium-mediated CC relaxation. It is to be mentioned that levels of serum lipids and testosterone did not change with pravastatin treatment in aged rats. These results support that preventive effect of pravastatin occur by a mechanism independent from the lipid-lowering effects of this drug. Similar observations were also previously reported [Citation44]. Moreover, in accordance with the previous studies [Citation45], long-term use of the pravastatin at the dose of 10 mg/kg did not affect blood pressure even though it improved age-related corporal dysfunction. This excludes possible contribution of improve in blood pressure in the pathophysiology of age-related CC dysfunction.
Because Rho is a major target of GGPP, inhibition of Rho and its downstream target, ROCK, is a likely mechanism to be mediating some of the pleiotropic effects of statins on cardiovascular disease [Citation46]. Therefore, the mechanism of its restorative effect against age-related CC dysfunction might be related with a possible decrease in Rho kinase activity. It has recently been reported that short-term statin therapy may lower RhoA/Rho kinase expression levels and improve cavernosal blood pressure response to ROCK inhibition and voltage-stimulation, and reversing an augmented vasoconstricted state associated with diabetes and/or hypertension in metabolic syndrome [Citation47]. We have therefore investigated whether its restorative effect occurs via preventing decreased eNOS and nNOS-mediated corporal relaxation, and by down-regulation of the ROCK vasoconstrictor anti-erectile system. Results of this study have shown that administration of pravastatin for six weeks to aged rats nearly normalized the eNOS and p-eNOS protein expression levels of the CC similar to the that of adult rats. Moreover, long-term pravastatin treatment caused downregulation of protein levels of RhoA and ROCK2. Inhibition of RhoA and ROCK2 expression with pravastatin treatment also paralleled with the reduction in CC dysfunction of aged rats. In addition, in our study, pravastatin treatment suppressed the increased gp91phox expression in aged CC, indicating that pravastatin could improve decreased NO-mediated corporal relaxation in aged penile tissue via suppression of gp91phox activity. Thus, the mechanism of protective action of this drug against ED may involve the normalization of NADPH oxidase activity. Taken together, the results presented in our study indicate that aging may cause CC dysfunction by overactivation of the gp91phox and Rho kinase, and treatment with pravastatin improves age-related CC dysfunction. These results suggested that protective effects of pravastatin on NO-mediated CC relaxation in aged rats might be associated with decreased NADPH oxidase and Rho kinase activity.
Interestingly, it has been reported that enhanced RhoA/Rho kinase pathway plays anti-erectile role and is associated with reduced response to PDE-5 inhibitor in diabetic animals [Citation48]. In addition, previous data from the literature reported that statins may ameliorate sildenafil-induced penile erections in experimental diabetes by inhibiting diabetes-induced RhoA/Rho kinase signaling hyperactivation [Citation49]. Thus, we also tested whether long-term pravastatin treatment would restore sildenafil-induced relaxation of CC in aged rat model of ED. Conclusively, pravastatin treatment showed effectiveness in restoring erectile responses of aged rats by possibly controlling the RhoA/Rho kinase pathway.
In conclusion, the results of this study provide first evidence for the combined effect of downregulation of the NADPH oxidase/Rho kinase and increased eNOS/nNOS levels contributing to therapeutic effect of pravastatin against age-related CC dysfunction. Moreover, results of this study indicate that long-term pravastatin treatment efficiently increased sildenafil citrate-induced relaxation of CC in aged rats. Based on these data, our findings provide a potential rationale for the clinical use of pravastatin in reducing age-related ED-independent from the lipid-lowering effects of this drug.
Declaration of interest
The authors report no declarations of interest.
This study was supported by Akdeniz University Research Foundation.
References
- NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 1993;270:83–90
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54–61
- Corona G, Lee DM, Forti G, et al; EMAS Study Group. Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS). J Sex Med 2010;7:1362–80
- Carrier S, Nagaraju P, Morgan DM, et al. Age decreases nitric oxide synthase-containing nerve fibers in the rat penis. J Urol 1997;157:1088–92
- Johnson JM, Bivalacqua TJ, Lagoda GA, et al. eNOS-uncoupling in age-related erectile dysfunction. Int J Impot Res 2011;23:43–8
- Musicki B, Kramer MF, Becker RE, Burnett AL. Age-related changes in phosphorylation of endothelial nitric oxide synthase in the rat penis. J Sex Med 2005;2:347–55; discussion 355–7
- Jin L, Liu T, Lagoda GA, et al. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J 2006;20:536–8
- Gao BH, Zhao ST, Meng FW, et al. Y-27632 improves the erectile dysfunction with ageing in SD rats through adjusting the imbalance between nNO and the Rho-kinase pathways. Andrologia 2007;39:146–50
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004;109:III39–43
- Nangle MR, Cotter MA, Cameron NE. Effects of rosuvastatin on nitric oxide-dependent function in aorta and corpus cavernosum of diabetic mice: relationship to cholesterol biosynthesis pathway inhibition and lipid lowering. Diabetes 2003;52:2396–402
- Gokce Mİ, Gülpınar Ö, Öztürk E, et al. Effect of atorvastatin on erectile functions in comparison with regular tadalafil use. A prospective single-blind study. Int Urol Nephrol 2012;44:683–7
- Trivedi D, Kirby M, Wellsted DM, et al. Can simvastatin improve erectile function and health-related quality of life in men aged ≥40 years with erectile dysfunction? Results of the Erectile Dysfunction and Statins Trial [ISRCTN66772971]. BJU Int 2013;111:324–33
- Mastalir ET, Carvalhal GF, Portal VL. The effect of simvastatin in penile erection: a randomized, double-blind, placebo-controlled clinical trial (Simvastatin treatment for erectile dysfunction-STED TRIAL). Int J Impot Res 2011;23:242–8
- Davignon J, Jacob RF, Mason RP. The antioxidant effects of statins. Coron Artery Dis 2004;15:251–8
- Dobrucki LW, Kalinowski L, Dobrucki IT, Malinski T. Statin stimulated nitric oxide release from endothelium. Med Sci Monit 2001;7:622–7
- Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998;97:1129–235
- Nakata S, Tsutsui M, Shimokawa H, et al. Statin treatment upregulates vascular neuronal nitric oxide synthase through Akt/NF-kappaB pathway. Arterioscler Thromb Vasc Biol 2007;27:92–8
- Ohkawara H, Ishibashi T, Saitoh S, et al. Preventive effects of pravastatin on thrombin-triggered vascular responses via Akt/eNOS and RhoA/Rac1 pathways in vivo. Cardiovasc Res 2010;88:492–501
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arterioscler Thromb Vasc Biol 2001;21:1712–9
- Coelho-Filho OR, De Luca IM, Tanus-Santos JE, et al. Pravastatin reduces myocardial lesions induced by acute inhibition of nitric oxide biosynthesis in normocholesterolemic rats. Int J Cardiol 2001;79:215–21
- Fontaine D, Fontaine J, Dupont I, et al. Chronic hydroxymethylglutaryl coenzyme A reductase inhibition and endothelial function of the normocholesterolemic rat: comparison with angiotensin-converting enzyme inhibition. J Cardiovasc Pharmacol 2002;40:172–80
- Kobayashi T, Matsumoto T, Kamata K. Mechanisms underlying the chronic pravastatin treatment-induced improvement in the impaired endothelium-dependent aortic relaxation seen in streptozotocin-induced diabetic rats. Br J Pharmacol 2000;131:231–8
- Burnett AL. Novel nitric oxide signaling mechanisms regulate the erectile response. Int J Impot Res 2004;16:S15–9
- Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol 2003;170:S6–13; discussion S13–14
- Rajasekaran M, White S, Baquir A, Wilkes N. Rho-kinase inhibition improves erectile function in aging male Brown-Norway rats. J Androl 2005;26:182–8
- Numao N, Masuda H, Sakai Y, et al. Roles of attenuated neuronal nitric-oxide synthase protein expression and accelerated arginase activity in impairing neurogenic relaxation of corpus cavernosum in aged rabbits. BJU Int 2007;99:1495–9
- Park K, Shin JW, Oh JK, et al. Restoration of erectile capacity in normotensive aged rats by modulation of angiotensin receptor type 1. J Androl 2005;26:123–8
- Park CS, Ryu SD, Hwang SY. Elevation of intracavernous pressure and NO-cGMP activity by a new herbal Formula in penile tissues of aged and diabetic rats. J Ethnopharmacol 2004;94:85–92
- Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 2003;284:R1–12
- Fulton D, Gratton JP, McCabe TJ, et al. Regulation of endothelium-derived nitric oxid production by the protein kinase Akt. Nature 1999;399:597–601
- Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J Pharmacol Exp Ther 2001;299:818–24
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 2003;83:1325--58
- Chang S, Hypolite JA, Changolkar A, et al. Increased contractility of diabetic rabbit corpora smooth muscle in response to endothelin is mediated via Rho-kinase beta. Int J Impot Res 2003;15:53–62
- Ming XF, Viswambharan H, Barandier C, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol 2002;22:8467–77
- Bivalacqua TJ, Champion HC, Usta MF, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA 2004;101:9121–6
- Silva FH, Mónica FZ, Báu FR, et al. Superoxide anion production by NADPH oxidase plays a major role in erectile dysfunction in middle-aged rats: prevention by antioxidant therapy. J Sex Med 2013;10:960–71
- Mason RP, Walter MF, Day CA, Jacob RF. Intermolecular differences of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors contribute to distinct pharmacologic and pleiotropic actions. Am J Cardiol 2005;96:11F–23F
- Jorge PA, Osaki MR, de Almeida E. Rapid reversal of endothelial dysfunction in hypercholeterolemic rabbits treated with simvastatin and pravastatin. Clin Exp Pharmacol Physiol 1997;24:948–95
- Jorge PA, Almeida EA, Ozaki MR, et al. Effects of atorvastatin, fluvastatin, pravastatin, and simvastatin on endothelial function, lipid peroxidation, and aortic atherosclerosis in hypercholesterolemic rabbits. Arq Bras Cardiol 2005;84:314–9
- Rakotoniaina Z, Guerard P, Lirussi F, et al. The protective effect of HMG-CoA reductase inhibitors against monocrotaline-induced pulmonary hypertension in the rat might not be a class effect: comparison of pravastatin and atorvastatin. Naunyn Schmiedebergs Arch Pharmacol 2006;374:195–206
- Guerard P, Rakotoniaina Z, Goirand F, et al. The HMG-CoA reductase inhibitor, pravastatin, prevents the development of monocrotaline-induced pulmonary hypertension in the rat through reduction of endothelial cell apoptosis and overexpression of eNOS. Naunyn Schmiedebergs Arch Pharmacol 2006;373:401–14
- Rao K, Liu JH. Cell apoptosis and male erectile dysfunction. Zhonghua Nan Ke Xue 2008;14:1126–9
- Kaneta S, Satoh K, Kano S, et al. All hydrophobic HMG-CoA reductase inhibitors induce apoptotic death in rat pulmonary vein endothelial cells. Atherosclerosis 2003;170:237–43
- de Sotomayor MA, Pérez-Guerrero C, Herrrera MD, et al. Improvement of age-related endothelial dysfunction by simvastatin: effect on NO and COX pathways. Br J Pharmacol 2005;146:1130–8
- Li C, Yang CW, Park JH, et al. Pravastatin treatment attenuates interstitial inflammation and fibrosis in a rat model of chronic cyclosporine-induced nephropathy. Am J Physiol Renal Physiol 2004;286:F46–57
- Laufs U, Endres M, Stagliano N, et al. Neuroprotection mediated by changes in the endotelial and cytosketketon. J Clin Invest 2000;106:15–24
- Wingard CJ, Moukdar F, Prasad RY, et al. Reversal of voltage-dependent erectile responses in the Zucker obese-diabetic rat by rosuvastatin-altered RhoA/Rho-kinase signaling. J Sex Med 2009;6:269–78
- Park K, Cho SY, Kim SW. Erectile response to type 5 phosphodiesterase inhibitor could be preserved with the addition of simvastatin to conventional insulin treatment in rat model of diabetes. Int J Androl 2011;34:e468–74
- Morelli A, Chavalmane AK, Filippi S, et al. Atorvastatin ameliorates sildenafil-induced penile erections in experimental diabetes by inhibiting diabetes-induced RhoA/Rho-kinase signaling hyperactivation. J Sex Med 2009;6:91–106
