Abstract
Objective: Lead exposure linked to osteoporosis in women. However, there is no direct evidence whether lead exposure has effects on bone metabolism in middle-aged male subjects. Therefore, the present study investigated the relationship between bone mineral densitometry measurements, bone markers, endocrine hormones and blood lead levels.
Material and methods: The present study included lead exposure patients (n: 30) and control subjects (n: 32). We recorded information on patient demographics and risk factors of osteoporosis. Blood lead levels were evaluated using Varian AA 240Z atomic absorption spectrophotometry. Bone mineral density measurements were measured using dual-energy X-ray absorptiometry.
Results: Each lumbar T and Z scores in the lead exposure group were lower than the control group. There were no significant differences in femur neck and femur total T and Z scores between two groups. Blood lead levels were also negatively correlated with lumbar 2-4 T score, total lumbar T score, lumbar 2-4 Z score and total lumbar Z score. Urinary hydroxyproline and urinary deoxypyridinoline levels in the lead exposure group were significantly higher compared to controls. Blood lead levels were strong, positively correlated with urinary deoxypyridinoline. Endocrine hormone levels and 1,25-dihydroxy-vitamin D3 levels were comparable between lead exposure and control group.
Conclusion: Lead exposure in male workers is an important factor for deterioration in bone mineral density. We should be screening blood lead levels and history of lead exposure in male osteoporosis.
Introduction
Chronic lead exposure is one of the most common occupational and environmental poisoning. Lead attaches to proteins in the blood that bring it to different tissues. Lead is stored in the blood, several organs and bones. Hematological, renal, liver and reproductive systems are often affected by lead exposure. Lead is stored in bone and can stay in the bone for years. Previous reports defined that lead had some complex and various effects on bone metabolism in women [Citation1]. However, there is limited information whether lead exposure has the effect on bone metabolism in male patients.
Chronic lead exposure, mainly two different mechanisms, can disturb the function of bone tissues. First, lead has a direct effect on the bone tissue. Several experimental and clinical studies demonstrated that lead exposure was related to reduction in bone mass, inhibition chondrocyte, osteoblast, osteoclast function, cell toxicity and apoptosis in mesenchymal stem cells [Citation2–5]. Second, lead exposures’ indirect effects cause distortion on bone tissues. Indirect effects are likely that contain several endocrine disorders. These cause worsening of bone mineral density [Citation6,Citation7]. In addition, lead exposure may cause diminished D vitamin activation and decreased calcium absorption from the gastrointestinal system [Citation8].
The association between lead exposure and bone metabolism has been widely investigated in women. These studies showed chronic lead exposure, postmenopausal status and older age significant risk factors of osteoporosis [Citation9–11]. Some authors argued that endogenous lead exposure is a potential risk factor for worsening bone mineral density in women [Citation12]. However, limited studies have focused on male subjects with lead exposure. Sun et al. evaluated bone resorption and formation markers in a lead exposure group. These authors detected that bone markers were elevated in male patients with lead exposure. However, the study showed that the bone mineral density measurements in the exposed group were lower than the control group without a significant statistical difference. Additionally, bone mineral density measurements in the high urinary lead level group were lower than the control group [Citation13]. On the other hand, other studies demonstrated that the occupational exposure to lead could cause the decline of the bone mineral density and the rise of the prevalence of lumbar vertebral fracture [Citation14–16]. A recent clinical study also shown in male patients that lead levels are related to osteoporosis [Citation17]. However, these studies usually include subjects of older age and there were no completely investigated etiological factors, such as; parathyroid hormone (PTH), total testosterone level, follicular stimulating hormone (FSH), luteinizing hormone (LH), thyroid stimulating hormone (TSAH) and 1,25-dihydroxy-vitamin D3.
Significant clinical guidelines screening of osteoporosis in men should investigate endocrine reasons [Citation18]. Lead exposure can also have endocrine effects. Therefore, our research investigated the relationship between blood lead levels and bone mineral densitometry measurement and bone markers and possible endocrine reasons.
Materials and methods
Study population
A total of 62 cases with lead exposure patients (n: 30) and control subjects (n: 32) were included in this study. The lead exposure group was randomly selected of subjects from lead poisoning that admitted to Ankara Occupational Diseases Hospital, between Februrary and July 2012. Their working had automobile lead and acid base storage battery. The control group was selected from security department in our hospital who were not exposed to lead in the work. The control group included 32 male subjects of equivalent age. All participants in this study were informed about the content and objectives of the study and gave their informed consent to participate. Local ethics committee gave permission to perform the study.
Subjects participating in the study filled in a questionnaire to obtain information on height, weight, age, cigarette smoking, alcohol use, socioeconomic status, job placement, work year, physical exercise, medical and drug history. Physical activity, daily calcium intake, daily tea and coffee consumption were recorded for all subjects. Body mass index (BMI) was calculated by using a formula; weight (kg)/height (m)2.
Exclusion criteria
Rheumatological diseases, neuronal diseases, endocrine disorders, liver, renal, cardiovascular diseases, malabsorption diseases, malign diseases, diabetes mellitus, anemia, alcohol consumption and depression treatment patients were excluded from this study. Cholestyramine, phenytoin, phenobarbital, heparin, rifampicin and corticosteroid using patients were also excluded. Indeed, subjects had risk factors for osteoporosis excluded as follows: low physical activities (physical activity or exercise <30 min per day), inadequate calcium intake (restricts the use of calcium and eliminates most dairy foods, all breads made with milk or dry skimmed milk and deep-green leafy vegetables), low BMI (<18 kg/m2), drinking excess tea (>4 cup per day), drinking excess coffee (>1 cup per day).
Biochemical tests (liver tests, renal test, electrolytes and thyroid tests), complete blood counts, PTH, total testosterone, FSH, LH and 1,25-dihydroxy-vitamin D3 were evaluated in all cases. Urinary calcium levels, urinary hydroxyproline and urinary deoxypyridinoline obtained from 24-h urine specimens. The blood lead levels were determined by the Ankara Occupational Diseases Hospital toxicology laboratory using Varian AA 240Z atomic absorption spectrophotometry (Palo Alto, CA).
Bone mineral density
Lumbar vertebrae (L2, L3, L4, L2-L4, total L) and femoral neck and femoral total scores are analyzed by using dual-energy X-ray absorptiometry (DEXA) instruments (QDR 1000; Hologic, Waltham, MA) in pencil-beam mo. Osteopenia was defined as a lowest T score −1.1 to −2.5 – and osteoporosis was defined as more than −2.5 SD.
Statistical methods
The results were shown as mean ± standard deviation and median (minimum–maximum) according to variable’s distribution. Qualitative variables were analyzed with the Chi-square test. Quantitative variables were analyzed with Student’s t-test and Mann–Whitney U-test. The Spearman correlation test was used to find the relationship between blood lead levels and BMD measurements. p Value less than 0.05 was considered statistically significant. SPSS 15.0 software (Chicago, IL) was used.
Results
In this study, a total of 53 male subjects were evaluated; 33 lead exposure group and 20 control subjects. The median age of the lead exposure group was found to be 37.9 ± 8.2 years and control groups median age was 39.4 ± 8.7 years. There is no significant difference between age within two groups (p = 0.138). Cigarette smoking frequency was found 50% and 70.1% in control and lead exposure group, respectively. This difference is statistically significant (p = 0.001). Liver test, renal function test, thyroid function test and whole blood counts were determined. Hemoglobin levels in the lead exposure group were significantly lower than the control group. As for the other biochemical measurements, no statistically significant difference was found between two groups. The levels of ALP in lead exposure groups were higher than that in the control subjects. However, this difference was not significant (p = 0.095). Possible risk factors for osteoporosis were analyzed as follows: 1,25-dihydroxy-vitamin D3, urinary calcium levels, parathyroid hormone, total testosterone level, follicular stimulating hormone, luteinizing hormone. No significant differences in the lead exposure group compared with the control group. Biochemical test results, whole blood count and levels of these factors are shown in .
Table 1. Biochemical test results shown in control and lead exposure groups.
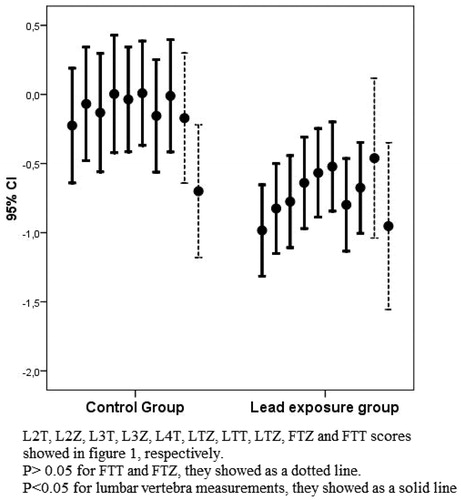
L2, L3, L4, L2-4, total lumbar, femur neck and total femur T and Z scores were evaluated. There was a significant difference in each lumbar T and Z scores between lead exposures groups and control group. However, there was no significant difference in femur neck and femur total T and Z scores between two groups. The results are summarized in and showed in . Osteopenia frequency in the lead exposure group was found to be 23.1% and osteoporosis frequency was 23.1%. Osteopenia frequency in control subjects was detected to be 27% and osteoporosis was not detected. Osteopenia frequency was similar between lead exposure and control group. However, osteoporosis frequency was found higher in the lead exposure group than the control group (p = 0.031). Blood lead levels in osteoporosis group was found to be 50.0 ± 21.6 μg/dL, osteopenia group was found to be 20.5 ± 17.8 μg/dL. The significant difference was detected to be p < 0.001).
Table 2. Bone mineral density measurements shown in control and lead exposure group.
The lead exposure group had high blood lead levels (45.9 ± 20 μg/dL) as compared to controls (1.9 ± 1.2 μg/dL). Lead levels were significantly different between two groups (p < 0.001). We observed a significant correlation between blood lead levels and some BMD measurements. Blood lead levels were negatively correlated with L2-4 T (r: −0.396, p: 0.008), total T (r: −0.341, p = 0.023), L2-4 Z (r: −0.389, p = 0.009) and total Z score (r = −0.380, p = 0.016). Other BMD parameters such as L2 T, L3 T, L2 Z, L3 Z, femur neck T and Z score were not significantly correlated with blood lead levels (p > 0.05).
Bone formation markers: alkaline phosphatase (ALP) and osteocalcin, bone resorption markers: urinary hydroxyproline and urinary deoxypyridinoline levels were measured. Although ALP levels were higher in the lead exposure group than the control group, there was a significant difference between two groups (p = 0.489). Similar to ALP, other bone formation marker as an osteocalcin levels were comparable between the control and the lead exposure group. Furthermore, bone resorption markers including urinary hydroxyproline, urinary deoxypyridinoline levels were elevated in the lead exposure group when compared to control subjects. Bone marker levels are shown in . Indeed, blood lead levels were strong, positively correlated urinary deoxypyridinoline (r: 0.685, p < 0.001), but there were no correlation blood lead levels and urinary hydroxyproline (p = 0.336). Although blood lead levels were not correlated with osteocalcin (p = 0.377), there was a positive correlation ALP levels (p = 0.005, r = 0.375).
Table 3. Bone formation and resorption marker values in control and lead exposure groups.
Discussion
We showed a clear association between blood lead levels and measurements of bone mineral density and bone resorption markers. However, formation markers are not affected by lead exposure. The current study found that lumbar vertebral bone mineral density measurements were affected by lead exposure. However, femur bone mineral density measurements were not affected. In addition, the results of this study did not show endocrine differences in the lead exposure group.
Bone tissue is the most accumulated site in chronic lead exposure. Lead exposure is a significant etiological factor for osteoporosis in women. Multiple reports showed the vital role of lead in osteopenia and osteoporosis [Citation9–11,Citation13,Citation16,Citation17]. However, there were few studies which evaluated the relationship between lead exposure and bone mineralization in male patients.
Our study showed that bone mineral density measurements especially lumbar vertebral levels (T and Z scores) were significantly lower in the lead exposure group when compared to control subjects. Femur bone mineral density measurements were similar to control subjects. The best part of previous reports between bone mineral density, bone markers and lead exposure were published by Sun et al. In 2007, Sun et al. published a paper in which they describe bone mineral density measurements in the lead exposure group were lower than that in the control group without a significant statistical difference [Citation13]. However, Sun suggests that the measurements were related to blood lead levels. This study demonstrated that BMD measurements were lower in patients with high lead levels. Sun et al. revealed that BMD was significantly decreased in the groups of the high urinary lead level compared with lower urinary lead levels with a significant difference in both genders, but no such significant difference was observed in the relationship between blood lead and BMD [Citation15]. In another study, Sun et al. found that the prevalence of osteoporosis would increase significantly with the increase of the urinary lead levels in a linear correlation manner [Citation16]. There was also such a significant relationship between blood lead and osteoporosis. A recent study by Raafat et al. found in male workers that elevated levels of lead concentration accompanied by osteoporosis when compared with control [Citation17]. The present study has shown that blood lead levels were significantly higher in patients with osteoporosis than the control subjects. It was also shown that blood lead levels were related to bone mineral density measurements in lumbar vertebral regions (both T and Z scores). Therefore, we think that blood lead levels are a significant factor in worsening bone mineral density in male patients.
Lead exposure is linked to bone mineral density measurements; also lead exposure is related to elevated fracture risk. The occupational exposure to lead could cause the increase in the prevalence of lumbar vertebral fracture [Citation14]. The present study also found that lumbar vertebral BMD measurements decreased. Therefore, the results of this investigation show that lead exposure may relate to lumbar vertebral fracture risk. The findings of the current study are consistent with those of Gerhardsson who found bone lead levels in cortical and trabecular bones in long-term-exposed lead smelter male workers [Citation19]. The high lead concentrations among the workers were observed in vertebrae.
There are different possible explanations for how lead exposure deteriorates bone mineral density. Several authors have demonstrated adverse effects of lead on both bone formation and resorption. There are relationships between lead and osteoblasts, osteoclasts and chondrocytes functions [Citation2–4]. Indeed, in vivo study showed that lead exposure associated with callus formation, maturation and remodeling [Citation20]. Lead could also induce cell toxicity and apoptosis in mesenchymal cells [Citation5]. Sun et al. also studied that in male patients bone markers were highly detected bone resorption markers in the lead exposure group [Citation13,Citation15,Citation16]. In the present study, we showed that bone resorption marker such as ALP, urinary hydroxyproline and urinary deoxypyridinoline levels were elevated in lead exposure groups. However, osteocalcin levels were similarly detected. We also showed that bone resorption markers were higher in the lead exposure group and blood lead levels were positively correlated. However, there is no association between osteocalcin and lead exposure. We think that lead exposure is mainly caused due to high bone turnover. Some authors argued that high bone turnover releases lead from its inactive state in bone hydroxyapatite crystals, which leads to an additional imbalance of bone resorption over-formation would occur from lead’s preferential toxic effects on osteoblasts [Citation20]. Previous studies showed that high environmental lead exposures among women currently going through menopause are at an additional risk of osteoporosis. This phenomenon is defined as an endogenous lead exposure. Also, the normative aging study demonstrated that endogen lead exposure was found in men [Citation8]. Both trabecular and cortical bone lead accumulation were seen.
Previous studies in male patients failed to consider the differing etiological factors of osteoporosis. Studies on the effects of lead on the endocrine system are mainly based on occupationally lead-exposed workers and experimental animal models [Citation6,Citation7]. Although evidence is conflicting, it has been reported that accumulation of lead affects the majority of the endocrine glands. In particular, it appears to have an effect on the hypothalamic-pituitary axis. Lead may interact with other factors in the course of postmenopausal osteoporosis, to aggravate the course of the disease, since lead is known to inhibit the activation of vitamin D and uptake of dietary calcium [Citation8]. However, in previous studies lead exposure caused to bone mineral density in male patients did not focus on endocrine factors. In our study, D vitamin, TSH, urinary calcium, FSH, LH, testosterone levels in the lead exposure group and the control group were similarly detected. It is possible to hypothesize that bone mineral density worsens independent endocrine factors.
In conclusion, lead exposure is related to bone mineral density partially lumbar vertebrae. Lead exposure is associated with high-bone resorption other than any etiological factors. We should investigate male osteoporosis blood lead levels and explore previous lead exposures.
Declaration of interest
We certify that no party having a direct interest in the results of the research supported this article or will confer a benefit on us or on any organization with which we are associated.
References
- International Programme on Chemical Safety (IPCS). Inorganic lead. Environmental Health Criteria 165. Geneva: World Health Organisation; 1995
- Zuscik MJ, Pateder DB, Puzas JE, et al. Lead alters parathyroid hormone-related peptide and transforming growth factor-beta1 effects and AP-1 and NF-kappaB signaling in chondrocytes. J Orthop Res 2002;20:811–18
- Angle CR, Thomas DJ, Swanson SA. Lead inhibits the basal and stimulated responses of a rat osteoblast-like cell line ROS 17/2.8 to 1 alpha,25-dihydroxyvitamin D3 and IGF-I. Toxicol Appl Pharmacol 1990;103:281–7
- Pounds JG, Long GJ, Rosen JF. Cellular and molecular toxicity of lead in bone. Environ Health Perspect 1991;91:17–32
- Sharifi AM, Ghazanfari R, Tekiyehmaroof N, Sharifi MA. Investigating the effect of lead acetate on rat bone marrow-derived mesenchymal stem cells toxicity: role of apoptosis. Toxicol Mech Methods 2011;21:225–30
- Doumouchtsis KK, Doumouchtsis SK, Doumouchtsis EK, Perrea DN. The effect of lead intoxication on endocrine functions. J Endocrinol Invest 2009;32:175–83
- Fortin MC, Cory-Slechta DA, Ohman-Strickland P, et al. Increased lead biomarker levels are associated with changes in hormonal response to stress in occupationally exposed male participants. Environ Health Perspect 2012;120:278–83
- Cheng Y, Willett WC, Schwartz J, et al. Relation of nutrition to bone lead and blood lead levels in middle-aged to elderly men. The Normative Aging Study. Am J Epidemiol 1998;147:1162–74
- Nash D, Magder LS, Sherwin R, et al. Bone density-related predictors of blood lead level among peri- and postmenopausal women in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2004;160:901–11
- Campbell JR, Auinger P. The association between blood lead levels and osteoporosis among adults – results from the third national health and nutrition examination survey (NHANES III). Environ Health Perspect 2007;115:1018–22
- Lee BK, Kim Y. Association between bone mineral density and blood lead level in menopausal women: analysis of 2008–2009 Korean National Health and Nutrition Examination Survey data. Environ Res 2012;115:59–65
- Hernández-Avila M, Smith D, Meneses F, et al. The influence of bone and blood lead on plasma lead levels in environmentally exposed adults. Environ Health Perspect 1998;106:473–7
- Sun Y, Jin TY, Sun DH, et al. Effects of occupational lead exposure on bone mineral density and bone metabolism in workers. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2007;25:257–62
- Sun Y, Sun DH, Zhu GY, et al. Effects of occupational lead exposure on lumbar vertebral fracture in male workers. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2007;25:645–58
- Sun Y, Sun D, Zhou Z, et al. Estimation of benchmark dose for bone damage and renal dysfunction in a Chinese male population occupationally exposed to lead. Ann Occup Hyg 2008;52:527–33
- Sun Y, Sun D, Zhou Z, et al. Osteoporosis in a Chinese population due to occupational exposure to lead. Am J Ind Med 2008;51:436–42
- Raafat BM, Hassan NS, Aziz SW. Bone mineral density (BMD) and osteoporosis risk factor in Egyptian male and female battery manufacturing workers. Toxicol Ind Health 2012;28:245–52
- Watts NB, Adler RA, Bilezikian JP, et al.; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:1802–22
- Gerhardsson L, Akantis A, Lundström NG, et al. Lead concentrations in cortical and trabecular bones in deceased smelter workers. J Trace Elem Med Biol 2005;19:209–15
- Carmouche JJ, Puzas JE, Zhang X, et al. Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency. Environ Health Perspect 2005;113:749–55