Abstract
Objective: We evaluated the safety of testosterone treatment and its efficacy on body composition in males with testosterone deficiency syndrome (TDS) over 24 months.
Methods: 50 males aged 50–65 years with TDS (Aging Males Symptoms Scale [AMS] > 26 and calculated free testosterone [cFT] 250 pmol/l) were administered 50 mg testosterone gel daily for one year. During the second year, patients received 1000 mg of testosterone undecanoate every 2–3 months. Outcome measures were clinical chemistry values and total testosterone; sex hormone-binding globulin and cFT, changes in AMS and International Prostate Symptom Score; and changes in body composition measured by dual-energy-x-ray absorptiometry.
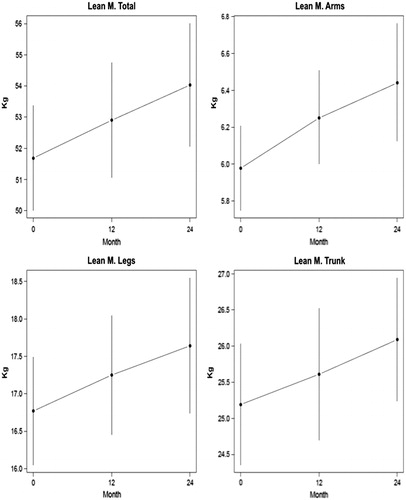
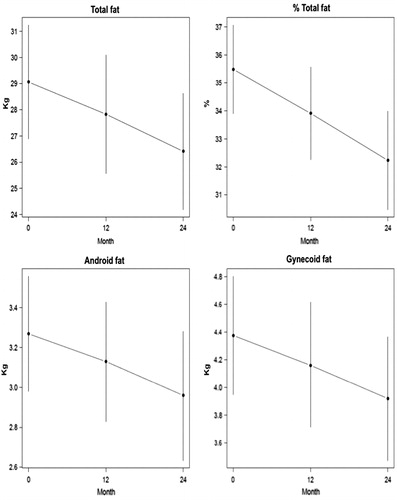
Results: There were no clinically significant changes in clinical chemistry safety parameters. There were significant improvements in both total and cFT and in AMS scores after three months (p < 0.001). Lean mass increased 2.35% at 12 months and 4.5% at 24 months, but proportionally more muscle mass was gained in arms and legs than in the trunk. Fat mass decreased 4.2% at 12 months and 9.1% at 24 months.
Conclusions: Testosterone treatment in males with TDS leads to body changes affecting lean and fat mass with significant improvement in AMS scores, and has an excellent safety profile.
Introduction
As men age, there is a gradual decline in free testosterone levels. At the age of 80 years, men only have 50% of the levels found in healthy 20-year-olds [Citation1,Citation2]. While total testosterone (TT) and albumin levels tend to fall, sex hormone-binding globulin (SHBG) levels increase. This leads to higher levels of serum testosterone binding to SHBG, resulting in decreased free and bioavailable testosterone fractions. Consequently, free and bioavailable testosterone levels fall with age to a greater extent than TT.
The decrease in serum testosterone levels has negative effects on sexual function and mood, also causing a decrease in bone mineral density and changes in body composition [Citation3–5]. With age, lean mass tends to decrease, while fat mass tends to increase. This process is enhanced when testosterone levels are low as a result of primary or secondary hypogonadism, also known as testosterone deficiency syndrome (TDS) [Citation6].
Changes in body composition have been widely found in primary and secondary hypogonadism, with changes that reverse upon treatment with testosterone: patients tend to regain lean mass and lose fat mass [Citation7]. In this study, we have assessed safety of treatment and changes in body composition and mass distribution after long term testosterone replacement therapy (24 months) in males with TDS.
Methods
Study design
This prospective, non-randomized, open-label, long-term follow-up study evaluated the safety and efficacy of testosterone treatment in men with TDS. TDS was defined as having a score >26 in the Aging Males Symptoms Scale [AMS] and a calculated free testosterone [cFT] 250 pmol/L. The study was carried out in Costa de Ponent, Spain, between July 2007 and April 2011. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. An independent Ethics Committee (Institutional Review Board) approved the study protocol, and all patients provided written informed consent prior to study participation.
Patients
Inclusion criteria were being male aged 50–65 years with TDS. Exclusion criteria included a previous diagnosis of primary or secondary hypogonadism, presenting chronic renal failure or bone metastasis, a history of congestive heart failure or recent angina, a previous cerebrovascular accident or untreated sleep apnea, having received treatment with androgens or treatment affecting bone mass such as bisphosphonates, calcium and vitamin D; being under therapy with gonadotropin-releasing hormone (GnRH) agonists and/or anti-androgens, presenting a prostate-specific antigen (PSA) >4 ng/ml or an International Prostate Symptom Score (IPSS) >16.
All patients received 50 mg of testosterone gel, applied in the morning, during the first year. Dose was adjusted either up to 75–100 mg or down to 25 mg, depending on safety and efficacy parameters. At the end of month 12, patients were given 1000 mg intramuscular testosterone undecanoate, followed by a second dose at the end of month 14. Successive doses were administered every 2–3 months until the last month of the study.
Measurements
The following measurements were performed on patients at baseline and at 3, 6, 9, 12, 18 and 24 months: systolic and diastolic blood pressure, heart rate, weight, height and body mass index (BMI); hematopoietic parameters (hemoglobin [Hb] and hematocrit [Hct]); glucose and glycosylated hemoglobin (HbA1C); transaminases and gamma-glutamyl transferase; total, high-density lipoprotein (HDL)- and low-density lipoprotein (LDL)-cholesterol; triglycerides; PSA, TT, albumin, SHBG and cFT. A single point plasma TT level measurement was performed between 07 and 11 h. TT was measured using a chemiluminescent immunoassay, and cFT was calculated following Vermeulen’s formula [Citation8]. The IPSS questionnaire and Spanish validated version of the AMS scale, consisting of 17 questions rated 1–5, were completed at each visit [Citation9,Citation10]. Dihydrotestosterone (DHT) and androstenediol gluconate levels were measured at baseline and at 6 and 12 months. Body composition, including total mass, lean mass and fat mass, as well as lean and fat mass distribution were measured by dual-energy-X-ray absorptiometry (DEXA) at baseline and at 12 and 24 months. DEXA scanning was performed using a Lunar Advanced 1 (GE Healthcare, Little Chalfont, UK) at CETIR Medical Group Regional fat distributions, such as android region fat, were obtained from the manufacturer’s software of regions of interest (ROI) analysis. This employs an algorithm that divides total body measurements into areas corresponding to head, trunk, arms and legs. “The android region” was defined as a lower boundary at the pelvis cut and the upper boundary above the pelvis cut by 20% of the distance between the pelvis and the neck cuts. The lateral boundaries are the arm cuts. The “gynecoid region” was defined as the boundary of umbilicus ROI to a distance equal to twice the height of the android fat region.
Statistical analysis
Quantitative variables were expressed as mean scores ± standard deviation (SD) and compared using the Student’s t-test. Statistical analyses were performed using SPSS v.17.0 statistical software package (SPSS Inc., Chicago, IL). Statistical significance was set at p < 0.05.
Results
Patients
Two hundred two men were initially screened, of whom 62 had TDS. Of them, 50 were eligible to participate in the study. There were two withdrawals during the first year, one due to elevated PSA levels and a diagnosis of prostate cancer and the other due to lung cancer. Three patients abandoned the study in the second year, two patients due to colon cancer and one patient due to personal reasons. shows baseline characteristics of patients. Mean age (SD) was 59.1 (1.3) (range 50–65) years. Mean TT level was 10.2 (3.6) nmol/l.
Table 1. Patient baseline characteristics.
Body composition results
There were no significant changes in total body mass over the course of the treatment: mean weight was 83.4 kg at baseline, 83.5 kg at 12 months and 83.3 kg at 24 months. Lean mass increased 2.3% after 12 months and 4.5% after 24 months (). Proportionally, there was a greater increase in lean mass in arms (4.6% and 7.7%) and legs (2.8% and 5.3%) at 12 and 24 months, respectively, compared to the trunk (1.6% and 3.5%) ().
Table 2. Changes in body composition (lean and fat body mass) after treatment with testosterone.
Testosterone replacement therapy induced significant reduction in total (−4.2% and −9.1%), android (−4.3% and −9.4%) and gynecoid (−4.81% and −10.5%) fat mass at 12 and 24 months, respectively. Although total fat mass and android fat decreased to a similar extent, the greatest decrease observed was in gynecoid fat ().
Hormone levels
Over the course of the study, concentrations of TT and cFT increased significantly from baseline values to normal value ranges (p < 0.001). The highest increases were found at 3 and 6 months, after which levels decreased slightly and then remained stable until the end of the study. Small fluctuations in SHBG levels were observed, but these were not statistically significant. DHT and androstenediol gluconate concentrations also increased significantly from baseline at all time-points. Since these measurements did not offer additional information besides that provided by TT and cFT, measurements were stopped at 12 months ().
Table 3. Changes in clinical, biochemical and hormonal parameters.
Other endpoints
There were significant improvements in the AMS questionnaire score from three months until the end of the study (p < 0.001). There were minimal significant changes in IPSS scores during the course of the treatment, but these were not considered clinically relevant ().
shows the clinical, biochemical and hormonal parameters at baseline and at months 12 and 24. No significant changes were observed during the study for metabolic parameters (total, HDL-, or LDL-cholesterol and triglycerides), glucose, HbA1C or for liver enzymes (data not shown). Hematopoietic parameters increased with testosterone replacement therapy and were statistically significant after 12 months (p < 0.001). At 12 months, Hb increased 0.9 g/dl versus baseline (p = 0.001) and the Hct by 2.9% (p = 0.001); at 24 months, Hb increased 1.6 g/dl versus baseline (p = 0.001) and Hct 4.2% (p = 0.001). Testosterone dose was reduced in a small number of patients (n = 7), specifically in those where Hb > 16.6 g/dl or Hct > 52% was detected. At 24 months, PSA levels increased significantly versus baseline (0.4 ng/ml), but this was not clinically relevant.
No treatment-related serious adverse events were observed. There were no withdrawals due to skin reactions at the application site. Three cases of mild gynecomastia resolved without treatment.
Discussion
In this study, we report long-term safety and efficacy data on lean mass, fat mass and safety over a 24-month period of testosterone replacement therapy in males with TDS. Compliance was excellent for a long-term study, with only five patients withdrawing and 90% completing. Although this was an open-label, long-term follow-up study with the possibility of dose adjustment, we believe that the data presented are relevant to the clinical practice of androgen replacement in males with TDS.
As reported in other studies, we found no significant changes in the levels of transaminases, gamma-glutamyltransferase, total, HDL- or LDL-cholesterol, or triglycerides [Citation4,Citation6]. Hb and Hct gradually increased from baseline. Although these increases were statistically significant, they were within safety margins, similarly to those reported in other studies [Citation4,Citation11]. In patients whose Hb was >17 g/dl or Hct was >52%, the dose of testosterone was reduced.
Some authors have reported no significant increases in PSA with testosterone treatment [Citation12,Citation13], while others have observed changes in both, the testosterone and placebo groups [Citation14]. We found significant increases in PSA (0.3 at month 12, p = 0.046 and 0.4 at month 24, p = 0.007), although these were considered of little clinical relevance. We had only one patient with raised PSA who was diagnosed with prostate cancer. This is a common occurrence in follow-up studies. For example, Dean et al. diagnosed three cases of prostate cancer among 300 patients within 9 months of follow up [Citation6].
There were minimal significant changes in IPSS scores during the course of the treatment, but these changes were not considered to be clinically relevant. Allan et al. reported similar results [Citation12], and Dean et al. found no significant changes in IPSS symptoms with urodynamic measurements, flow meters and residual volumes [Citation6]. Also comparable to similar studies, there were no variations in either body weight or BMI [Citation14].
Testosterone levels increased 88% after 12 months treatment, stabilizing afterwards, which is in accordance to what has been observed by other authors after 6 months treatment [Citation5,Citation15,Citation16]. Also as reported by other authors, there were no significant changes in SHBG [Citation4]. During the first year, we measured DHT and androstenediol gluconate and found that they had increased significantly, a result which has also been reported elsewhere [Citation4].
We used the AMS scale [Citation17] to assess health-related quality of life and clinical improvement in our patients and found that improvement occurred progressively over the course of the study. In the AMS questionnaire, there was particularly marked improvement in the items related to sexual function, an important aspect that has frequently been noted [Citation5,Citation15,Citation18]. We also found improvement in mood status, a result also reported by other authors [Citation19].
We have not been able to confirm these changes in a placebo group, and some of the benefits of testosterone treatment could be attributed to a placebo effect. However, the fact that these changes in behavior were sustained throughout the study would suggest otherwise. Moreover, it is evident that improvements in sexual function and mood increased quality of life, as confirmed by improvement in the AMS scale.
Previous studies have evaluated the effects of testosterone treatment on body composition. Bhasin et al. concluded that older men are as sensitive as young men to the effects of testosterone treatment on skeletal muscle. They prescribed testosterone to 60 men aged 60–75 years and to 61 men aged 19–35 years, who had previously been treated with GnRH to suppress the production of androgens. The authors found that the two groups responded equally well to testosterone treatment [Citation13]. A 12-month study on men aged 21–81 years found increases in lean mass of 1.7 kg and 2.2 kg and decreases in fat mass of −1.2 kg and −1.8 kg at 6 months and 12 months, respectively [Citation6].
Wang et al. observed similar results treating a group of patients aged 19–68 years over 36 months. Patients experienced an increase in muscle mass of 2.0 kg at 6 months, 2.9 kg at 18 months and 2.9 kg at 30 months, while fat mass decreased by −0.8 kg at 6 months, −1.6 kg at 18 months and −1.30 kg at 30 months. No relevant changes were found beyond 24 months [Citation4]. These results are similar to our findings, although our patients had late-onset hypogonadism and were limited to a more narrow age range (50–65 years).
Other researchers have assessed testosterone treatment in older hypogonadal patients aged over 65 years. Srinivas-Shankar et al. found increases in lean mass and decreases in fat mass in this age group [Citation20]. They treated these patients for 6 months and then followed them up for a further six months without treatment. After follow-up was completed, it was observed that all progress that the patients had been made in the first six months had been lost after stopping treatment [Citation21]. One long-term 36-month study in males aged over 65 years found increases in lean mass and decreases in fat mass, with a notably greater increase in trunk lean mass compared with arms and legs [Citation22]. Our results showed the opposite effect, with a greater percentage increase in arms and legs than in trunk.
Our study findings are in accordance with those of Allan et al. in a population of non-obese patients of similar age (mean age 62.1 years), where a significant percentage increase in lean mass in arms and legs was reported, with less significant increase in lean mass in trunk. A decrease in visceral fat was also reported [Citation12]. However, changes in subcutaneous abdominal adipose mass were not reported, which would correspond to android fat noted in our study. This is probably due to the fact that participants were non-obese and therefore without excess abdominal fat at baseline.
Some articles published recently show similar effects of testosterone on body composition in patients with symptomatic testosterone deficiency [Citation23,Citation24]. The improvements reported in this study add further evidence to the benefits of testosterone replacement therapy in men with TDS, where significant improvements in lumbar spine and hip bone mineral density have been reported [Citation25].
Testosterone replacement therapy in men with TDS causes improvements in body composition, increasing lean mass, primarily in arms and legs, and decreasing fat mass overall, and to the greatest extent in the android and gynecoid regions. Testosterone treatment also improves ageing-related symptoms, as assessed by the AMS questionnaire. A significant but non-relevant increase in PSA was noted, as well as a significant increase in erythropoiesis, which remained within safety margins.
Declaration of interest
The authors have no conflicts of interest to declare.
References
- Purifoy FE, Koopmans LH, Mayes DM. Age differences in serum androgen levels in normal adult males. Hum Biol 1981;53:499–511
- Deslypere JP, Vermeulen A. Leydig cell function in normal men: effect of age, life-style, residence, diet, and activity. J Clin Endocrinol Metab 1984;59:955–62
- Mellstrom D, Johnell O, Ljunggren O, et al. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res 2006;21:529–35
- Wang C, Cunningham G, Dobs A, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 2004;89:2085–98
- McNicholas TA, Dean JD, Mulder H, et al. A novel testosterone gel formulation normalizes androgen levels in hypogonadal men, with improvements in body composition and sexual function. BJU Int 2003;91:69–74
- Dean JD, Carnegie C, Rodzvilla J, Smith T. Long-term effects of testim(r) 1% testosterone gel in hypogonadal men. Rev Urol 2004;6:S22–9
- Katznelson L, Finkelstein JS, Schoenfeld DA, et al. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 1996;81:4358–65
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72
- Heinemann LAJ, Zimmerman T, Vermeulen A, et al. A new ‘aging males’ symptoms rating scale. Aging Male 1999;2:105–14
- Mas M, EFA Study Group. Psychometric validation of the Spanish version of the Ageing Males’ Symptoms Scale (AMSS) in a population-based sample. J Sex Med 2008;5:106
- Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab 1999;84:1966–72
- Allan CA, Strauss BJ, Burger HG, et al. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab 2008;93:139–46
- Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 2005;90:678–88
- Kenny AM, Prestwood KM, Gruman CA, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 2001;56:M266–72
- Steidle C, Schwartz S, Jacoby K, et al. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab 2003;88:2673–81
- Wang C, Swerdloff RS, Iranmanesh A, et al. Effects of transdermal testosterone gel on bone turnover markers and bone mineral density in hypogonadal men. Clin Endocrinol (Oxf) 2001;54:739–50
- Heinemann LA, Saad F, Heinemann K, Thai DM. Can results of the Aging Males’ Symptoms (AMS) scale predict those of screening scales for androgen deficiency? Aging Male 2004;7:211–8
- Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000;85:2839–53
- Khera M, Miner M, Bhattacharya R, et al. Testosterone supplementation significantly improves depression symptoms in hypogonadal men enrolled in the TESTIM registry in the US (TRIUS). J Urol 2010;183:e578
- Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 2010;95:639–50
- O’Connell MD, Roberts SA, Srinivas-Shankar U, et al. Do the effects of testosterone on muscle strength, physical function, body composition, and quality of life persist six months after treatment in intermediate-frail and frail elderly men? J Clin Endocrinol Metab 2011;96:454–8
- Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 1999;84:2647–53
- Behre HM, Tammela TL, Arver S, et al. A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male 2012;15:198–207
- Bouloux PM, Legros JJ, Elbers JM, et al. Effects of oral testosterone undecanoate therapy on bone mineral density and body composition in 322 aging men with symptomatic testosterone deficiency: a 1-year, randomized, placebo-controlled, dose-ranging study. Aging Male 2013;16:38–47
- Rodríguez-Tolrà J, Torremadé J, Di Gregorio S, et al. Effects of testosterone treatment on bone mineral density in men with testosterone deficiency syndrome. Andrology 2013;1:570–5