Abstract
Purpose: We examined the prevalence of low testosterone (LT) and its relationship with body mass index (BMI) in men with lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH), who were enrolled in a clinical trial of drug therapy, the Medical Therapy of Prostatic Symptoms (MTOPS) Study.
Materials and methods: MTOPS enrolled 3047 men, and of these, 1896 had total testosterone (TT) measured at baseline. LT was defined as a single measurement of TT of <300 ng/dL.
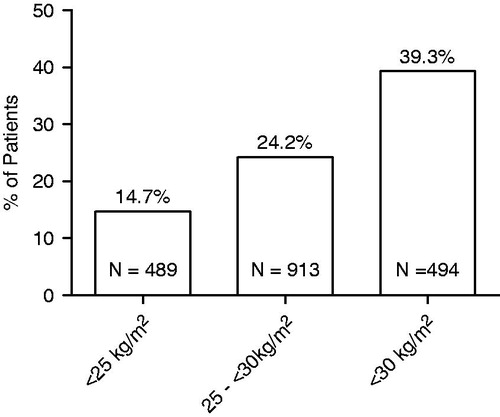
Results: The overall prevalence of LT was 25.7%. Prevalence increased with increasing BMI; 14.7% among men who were normal weight (BMI <25 kg/m2) and 24.2% and 39.3% among overweight (BMI 25 to <30 kg/m2), and obese (baseline BMI ≥30 kg/m2) men, respectively.
Conclusions: LT was observed in about one in four MTOPS study participants with baseline TT measurements. The prevalence of LT increased markedly with increasing BMI. Our findings suggest a high prevalence of LT in obese men with LUTS/BPH. Physicians should be alert to the possibility of symptoms of hypogonadism in this population.
Introduction
Hypogonadism in men is a clinical syndrome that results from failure of the testes to produce physiological levels of testosterone (androgen deficiency) and a normal number of spermatozoa due to disruption of one or more levels of the hypothalamic-pituitary-testicular (HPT) axis. Symptoms of hypogonadism are associated with low levels of serum total testosterone (TT) (<300 ng/dL) [Citation1]. It is well established that increased body mass index (BMI) is a risk factor for both benign prostatic hyperplasia (BPH) and low testosterone levels (LT) in aging men [Citation2–4]. However, to date, only two small studies have examined the prevalence of low testosterone levels in BPH patients. Each of these studies found that approximately one in five men had LT; with an increased prevalence in obese men [Citation5,Citation6]. We examined the prevalence of LT (here defined as TT <300 ng/dL) and the relationship between LT and BMI in a large subset of men enrolled in the Medical Therapy of Prostatic Symptoms (MTOPS, NCT00021814) trial, a clinical study to determine the effects of long-term medical therapy on clinical progression of BPH in men with lower urinary tract symptoms (LUTS) secondary to BPH.
Methods
The MTOPS trial enrolled men at least 50 years of age with an American Urological Association Symptom Index score of 8–30 (8–35 in the pilot phase), a maximum urinary flow rate (Qmax) of 4–15 mL/s and a voided volume of at least 125 mL. A total of 3047 men were enrolled, including 116 from the pilot study. We studied baseline TT data from 1198 of these men who participated in a sub-study that included measurement of hormones and a prostate biopsy at baseline and at pre-determined time points during follow-up [Citation7]. We also examined baseline TT data from an additional 698 MTOPS patients (not included in the sub-study described earlier) who participated in the full-phase study and were independently evaluated for TT at baseline by trial physicians. Thus, the overall MTOPS TT analysis population included a total of 1896 men.
We defined men with LT as having a single measurement of TT <300 ng/dL. Men were grouped according to the following categories of BMI: <25 kg/m2 (normal weight), ≥25 to <30 kg/m2 (overweight) or ≥30 kg/m2 (obese) and the percentage of patients with LT in each BMI group was determined.
Serum testosterone was measured using a radioimmunoassay kit (Testosterone Coat-A-Count) manufactured at that time by DPC (Los Angeles, CA, USA) now available from Cruinn Diagnostics Limited (Dublin, Ireland). BMI was calculated as (weight (lb)/[height (in)]2) × 703.
Results
Characteristics of study participants
Selected baseline characteristics of the 1896 men who had TT measured at baseline are shown in . Overall, they had a mean baseline TT of 397.4 ng/dL, a mean BMI of 27.7 kg/m2 and a mean age of 62.6 years; 25.8% were normal body weight, 48.1% were overweight and 26.1% were obese. The mean age and BMI of the subgroup analyzed here was similar to the remaining men enrolled in the trial who did not have TT measurements (data not shown). Men with LT (baseline TT<300 ng/dL) had a mean baseline TT of 235.6 ng/dL, mean BMI of 29.6 kg/m2 and mean age of 62.7 years. Men with normal baseline TT (baseline TT ≥ 300 ng/dL) had a mean baseline TT of 453.4 ng/dL, mean BMI of 27.1 kg/m2 and mean age of 62.5 years. Men in each of the three BMI categories examined in this analysis (normal weight, overweight and obese) were similar with respect to mean age.
Table 1. Patient population characteristics.
Prevalence of low testosterone levels
Three-fourth (75%) of MTOPS participants we studied were overweight or obese. Overall, one-fourth (25.7%) had LT. The prevalence of LT increased with increasing BMI; it was 14.7% in normal weight men, 24.2% in overweight men and 39.3% in those who were obese (). Among the 487 men with LT, most (85%) were overweight or obese.
Discussion
Among aging men with LUTS secondary to BPH, about one in four were found to have LT. The prevalence of LT we observed in a group of men selected for LUTS/BPH is within the range of prevalence reported for the general male population >30 years of age [Citation8–11], though at least one study found the prevalence of LT in the general population to be substantially lower [Citation12]. We observed the highest prevalence of LT in obese LUTS patients (39.3%), with decreasing prevalence in overweight (24.2%) and normal weight (14.7%) patients. The increased prevalence of LT in obese patients was similar to that observed in the Proscar Long-Term Efficacy and Safety Study, in which 36.1% of 61 obese BPH patients had LT at baseline [Citation6]. The well-established relationship between LUTS/BPH and increased BMI [Citation2–4] was reconfirmed in our study as the majority (75%) of men were overweight or obese.
The results of this analysis are consistent with previous work in which LT was observed to be associated with increased BMI [Citation6,Citation13–17], or with waist circumference [Citation18]. There are a number of mechanisms that may explain the inverse relationship between higher BMI and testosterone levels in men. Adipose tissue is the main peripheral source of aromatase, which catalyzes the irreversible conversion of T to estradiol. The increased adiposity in obesity may be associated with increased aromatase activity, which could lead to an increase in the conversion of T to estradiol [Citation19]. Obese men have been shown to have elevated serum estradiol levels [Citation20]. Increased estradiol levels may, in turn, cause pituitary suppression [Citation19], which may explain, at least in part, the reduction in gonadotropin release reported in obese men [Citation19,Citation20]. Obesity has also been shown to be associated with reduced sex hormone-binding globulin levels, which could lead to a further reduction in serum testosterone levels [Citation20,Citation21].
In light of the role testosterone plays in maintaining male sexual function [Citation22,Citation23], together with its roles in regulation of bone density, muscle mass and function, fat mass and cardiovascular fitness [Citation24,Citation25], physicians should be aware of the relatively high prevalence (39.3%) of LT we observed in obese men with LUTS secondary to BPH. Consideration might also be given to the possibility that the symptoms associated with LT could exacerbate the significant deterioration in quality of life associated with LUTS [Citation26,Citation27]. Practice guidelines [Citation1] for men with significant symptoms of androgen deficiency and LT include testosterone replacement therapy. However, physicians managing LUTS patients with LT are presented with a possible dilemma. It has been reported that in some patients testosterone therapy can lead to significant increases in both prostate volume and prostate-specific antigen (PSA) [Citation28–32]. As both prostate volume and serum PSA levels positively correlate with acute urinary retention and BPH-related surgery [Citation33,Citation34] there is concern that a stimulatory effect of testosterone therapy on the prostate could worsen urinary symptoms and offset the beneficial effects of medical therapies for LUTS/BPH (alpha-blockers and 5α-reductase inhibitors). Moreover, clinically significant elevations in serum PSA reported with some testosterone therapies [Citation28] may complicate the interpretation of PSA levels in men using 5α-reductase inhibitors. However, in studies that evaluated the effect of testosterone replacement therapy on urinary symptoms, no changes were noted [Citation29–31]. Furthermore, a meta-analysis of testosterone supplementation trials [Citation35] concluded that therapy does not increase either prostate size or PSA levels, or worsen urinary function.
Our findings that men with LUTS secondary to BPH exhibit a relatively normal range of serum testosterone, rather than being skewed towards higher levels, are consistent with the observations made in the meta-analysis [Citation35]. However, as there may be variation among individuals in response to testosterone replacement therapy, particularly with regard to prostate volume changes [Citation32], physicians administering testosterone replacement to patients with LT should consider routinely monitoring prostate and urinary status during therapy [Citation31].
Limitations of this analysis include the non-random nature of the study population, the cross-sectional study design, use of a single measure of testosterone, the limited racial/ethnic composition of the study group (mostly Caucasian), likely variability in the time of day a blood sample was collected, and absence of information on clinical status (symptoms related to low testosterone).
Conclusions
In conclusion, the present analysis of baseline TT data from 1896 men participating in the MTOPS trial demonstrated that about one in four LUTS patients, and greater than one in three obese LUTS patients, had LT. In light of these findings, physicians may want to consider examining men with LUTS/BPH for concomitant androgen deficiency.
Declaration of interest
S.A.K. has received compensation from Merck & Co., Inc. A.G.M. and E.A.O. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA, and may hold stock/stock options in the company. J.W.K. and J.Y.L. have no disclosures to report. MTOPS was supported by the following cooperative agreements from the National Institute of Diabetes, Digestive and Kidney Diseases: U01 DK49977, U01 DK46416, U01 DK41418, U01 DK46429, U01 DK46431, U01 DK46437, U01 DK46468, U01 DK46472, U01 DK49880, U01 DK 49912, U01 DK49921, U01 DK49951, U01 DK49954, U01 DK49960, U01 DK49961, U01 DK49963, U01 DK49964, U01 DK49971, U01 DK49980, as well as by the National Center for Minority Health and Health Disparities, NIH. Financial support and drug products for MTOPS were also provided by Merck and Pfizer.
Acknowledgements
The authors wish to acknowledge the contributions of the many investigators, study coordinators and patients who contributed to the MTOPS trial. Editorial assistance was provided by Jennifer Rotonda, Ph.D. of Merck & Co., Inc., Whitehouse Station, NJ, USA.
References
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536–59
- Giovannucci E, Rimm EB, Chute CG, et al. Obesity and benign prostatic hyperplasia. Am J Epidemiol 1994;140:989–1002
- Lee RK, Chung D, Chughtai B, Te AE, Kaplan SA. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int 2012;110:540–5
- Wang S, Mao Q, Lin Y, et al. Body mass index and risk of BPH: a meta-analysis. Prostate Cancer Prostatic Dis 2012;15:256--72
- Schatzl G, Brossner C, Schmid S, et al. Endocrine status in elderly men with lower urinary tract symptoms: correlation of age, hormonal status, and lower urinary tract function. The Prostate Study Group of the Austrian Society of Urology. Urology 2000;55:397–402
- Kaplan SA, O’Neill E, Lowe RS, et al. Testosterone in aging men with benign prostatic hyperplasia: data from the Proscar Long-term Efficacy and Safety Study (PLESS). Aging Male 2013;16:38--47
- Bautista OM, Kusek JW, Nyberg LM, et al. Study design of the Medical Therapy of Prostatic Symptoms (MTOPS) trial. Control Clin Trials 2003;24:224–3
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007;92:4241–7
- Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2001;86:724–31
- Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006;60:762–9
- Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123–35
- Rohrmann S, Platz EA, Selvin E, et al. The prevalence of low sex steroid hormone concentrations in men in the Third National Health and Nutrition Examination Survey (NHANES III). Clin Endocrinol (Oxf) 2011;75:232–9
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–9
- Kaplan SA, Meehan AG, Shah A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively high incidence of erectile dysfunction observed in these men? J Urol 2006;176:1524–7
- Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004;27:1036–41
- Laaksonen DE, Niskanen L, Punnonen K, et al. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. J Clin Endocrinol Metab 2005;90:712–9
- Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci 2002;57:M76–99
- Hall SA, Esche GR, Araujo AB, et al. Correlates of low testosterone and symptomatic androgen deficiency in a population-based sample. J Clin Endocrinol Metab 2008;93:3870–7
- Cohen PG. The hypogonadal-obesity cycle: role of aromatase in modulating the testosterone-estradiol shunt – a major factor in the genesis of morbid obesity. Med Hypotheses 1999;52:49–51
- Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab 1993;76:1140–6
- Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J Clin Endocrinol Metab 1996;81:1821–6
- Aversa A, Isidori AM, De Martino MU, et al. Androgens and penile erection: evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunction. Clin Endocrinol (Oxf) 2000;53:517–22
- Foresta C, Caretta N, Rossato M, et al. Role of androgens in erectile function. J Urol 2004;171:2358–62, quiz
- Makhsida N, Shah J, Yan G, et al. Hypogonadism and metabolic syndrome: implications for testosterone therapy. J Urol 2005;174:827–34
- Gooren LJ. The age-related decline of androgen levels in men: clinically significant? Br J Urol 1996;78:763–8
- Roberts RO, Jacobsen SJ, Rhodes T, et al. Natural history of prostatism: impaired health states in men with lower urinary tract symptoms. J Urol 1997;157:1711–17
- Girman CJ, Jacobsen SJ, Tsukamoto T, et al. Health-related quality of life associated with lower urinary tract symptoms in four countries. Urology 1998;51:428–36
- U.S. prescribing information for ANDROGEL® (testosteron gel), April 2011. FDA. 4-1-2011. 8-16-2012. Ref Type: Electronic Citation
- Behre HM, Bohmeyer J, Nieschlag E. Prostate volume in testosterone-treated and untreated hypogonadal men in comparison to age-matched normal controls. Clin Endocrinol (Oxf) 1994;40:341–9
- Marks LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA 2006;296:2351–61
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004;350:482–92
- Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E. Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic study. J Clin Endocrinol Metab 2003;88:2049–54
- Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 1999;53:473–80
- Roehrborn CG, Malice M, Cook TJ, Girman CJ. Clinical predictors of spontaneous acute urinary retention in men with LUTS and clinical BPH: a comprehensive analysis of the pooled placebo groups of several large clinical trials. Urology 2001;58:210–16
- Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 2005;60:1451–7