Abstract
Erectile dysfunction develops among 46.2% of men between 40 and 70 years. Studies demonstrated substitution on detrusor muscle by collagen due testosterone deprivation. It is clear the correlation among aging and oxidative stress, accelerating apoptosis process in many tissues. This study aims to demonstrate the collagen substitution over the muscle fibers on muscle structure of rat’s penis and the effects of testosterone supplementation. Sixteen senescent Wistar rats were divided into two groups: treatment (receiving standard supplementation testosterone dose) and control (receiving equivalent saline solution). Testosterone was dosed on D0 and D56 of study. All penises were prepared with picrosirius colored histology; stereology was applied to determine the volumetric density of collagen fibers (Vv). Analysis of variance demonstrated testosterone group’s replacement therapy to be effective, while the androgenic decline continued by the time of experiment in control group (p < 0.05). Testosterone group had Vv of 20.6%, lower than control group (47.8%); t-test (p < 0.001). Pearson’s correlation demonstrated an inverse correlation between the Vv and testosterone’s levels (p < 0.001). This is a pioneer study on demonstration of structural alterations over the cavernous corpora muscle caused by deprivation of testosterone on elderly rat. These finding implicate that the testosterone levels can influence, not only the libido, but also the erectile function.
Introduction
Erectile dysfunction is directed linked to age and it is the main well established risk factor for the appearance of erection difficulties in men [Citation1]. Its prevalence is 46.2% on Brazilian male population between 40 and 70 years old, and it causes a great social, psychological and physical impact [Citation2]. Age, expressed as androgen deficiency of the adult male and plurimetabolic syndrome, expressed on obesity, are the most known organic causes [Citation3]. However, such clinical finding may be explained the alteration of cavernous corpora muscle fiber composition and distribution during the aging process [Citation4]. A similar process can be observed over the aged bladder caused by the deprivation of testosterone [Citation5].
Disequilibrium in the pro- and anti-apoptosis mechanism is critical in the aging process [Citation6]. Apoptosis plays a role in various mitotic tissues, as in the liver and leucocytes, as it prevents tumorigenesis and keeps track of immunocompetent cells [Citation7]. However, in post-mitotic cells, as those in the penile muscles and bladder detrusor muscle, it has a negative effect since there is destruction of essential [Citation8,Citation9] and sometimes irreplaceable cells [Citation10].
Recent studies have shown that oxidative stress can alter the structure and function of the bladder in mice. Dambros et al. [Citation11] showed that bladder strips subjected to repeated electrical stimulation are involved with reduction of contractile force and no apparent increase of oxidants, suggesting that hydrogen peroxide forms induced cell damage depending on the concentration of the oxidants generated. Frequently, antioxidant mechanisms are able to limit or prevent the adverse effects of hydrogen peroxide, but with age, these mechanisms decrease, thereby making oxidative damage more prevalent with aging [Citation12].
Lorenzetti [Citation13] showed that fibrosis of the bladder wall was higher within the orchiectomy group, probably resulting from the sharp drop and not adaptive levels of testosterone, thus losing the ability to differentiate into smooth muscle stroma and anti-inflammatory power. However, apoptosis in the detrusor muscle was stronger in senescent rats, perhaps by greater involvement of free radicals when androgen decline is slow.
The genitourinary tract is especially sensitive to changes in serum testosterone levels. The versatility of testosterone and its interference in various chains of action in the bladder was first observed by Holgman [Citation14]. Studies in the basic science demonstrated the close relation between dihydrotestosterone (DHT) receptor in the bladder muscle and suppression of detrusor activity [Citation15,Citation16].
The importance of testosterone on bladder physiology and its decrease with aging have encouraged research on the impact of these phenomena on the genitourinary tract detrusor muscle structure apoptosis. Nakazawa et al. [Citation17], demonstrated, for the first time, increased expression of angiotensin-converting enzyme and angiotensin receptor type II (AII) in the bladder of rats undergoing bilateral orchiectomy. Fraga-Silva et al. [Citation18] showed that the use of one angiotensin inhibitor reduces penile fibrosis associated with attenuation of oxidative stress. Kilarkaje et al. [Citation19] demonstrated that Angiotensin II signaling is involved in diabetes-induced structural changes on penile structure. Since angiotensin type II acts through the rho/rho kinase via, this means that there are increasing pro-apoptotic factors associated with declining levels of testosterone.
Also, it is known that the production of Nitric Oxide (NO) in the urinary tract depends on the regulation of testosterone, and its potential relaxing effect of the detrusor muscle and the bladder neck can be impaired in hypogonadic patients [Citation20]. Sánches et al. [Citation21] demonstrated that nitrergic dysfunction and impaired neural NO signalling due to oxidative stress and nNOS uncoupling in penile arteries under conditions of insulin resistance.
There are data in the literature that describe the changes over the male penis muscle architecture during the aging process or over its effects on the physiopathology of erectile dysfunction.
Regarding what was previous discussed, the objective of this work is to study the relationship between late onset hypogonadism and structural remodeling of the senescent penile muscle structure, and whether this process can be influenced by exogenous testosterone replacement. Once there is enough information about the changes over the bladder detrusor muscle caused by oxidative stress, decreasing of testosterone and, by consequence, aging, it is logical to think that alterations of this nature can be observed in penile muscle structure and could lead to erectile dysfunction.
Materials and methods
Sixteen male senescent Wistar senile rats (18–20 months old), weighing 380–530 g were kept in a controlled environment (25 ± 2 °C) with exposure to light for 12 h a day, water available ad libitum and Labina® animal chow (Purina®). The study was carried out according to the guidelines of the Brazilian College for Animal Experimentation (COBEA) under approval of the Institutional Committee for Ethics in Animal Research.
Two groups were formed, double-blinded, as follows: Testosterone Group – 10 senile animals subjected to testosterone supplementation: Control Group – 6 senile animals subjected to intramuscular injection of 0.9% saline solution. This sample was statistically calculated with a magnitude of effect of 2.1 (99% of the subjects in the experimental group will exceed the mean value of the control group).
All animals were anesthetized with a solution composed of Xylazine (0.87 mg/kg) and Ketamine (43.3 mg/kg) injected intraperitoneally. After anesthesia was reached, they were weighed and later the venous blood was held through puncture of the retro-orbital venous plexus with a Pasteur pipette [Citation22], transferring the blood sample directly to an Eppendorf tube. Subsequently, the sample was centrifuged and only the serum was frozen at −20 °C.
Testosterone undecanoate (TU) was administered intramuscularly (50 mg/kg) into the animal’s dorsum using a fine insulin type needle, on the 1st and 28th day of the experiment. In the control group, 0.9% saline was administered at the same volume of drug solution in the testosterone group, on the same days. On the last day of the experiment (56th day), new blood sample was collected and the penis was removed by a section over its base. After removal, the penile were stored in formalin for 48 h and then in 70% alcoholic solution for further paraffinization and study of stereological collagen fibers.
Stereology was the method chosen in order to evaluate morphometrically the muscle and collagen fibers of penis. The fiber analysis was performed using preparations from 5-µm thin sections [Citation23]. The modified picrosirius red staining technique was used. The slides were analyzed via optical microscopy with polarized light at a 40 × magnification. Ten fields per slide and 10 slides per animal were evaluated. The volumetric density of the collagen and muscle fibers was analyzed by overlaying the M-42 grid system on the digital morphological image of the slides. The volumetric density was the relative density taken up by fibers in the tissue under examination. The stereological method determined quantitatively the parameters of the anatomical structural base on the two-dimensional thin sections, in three dimensions [Citation24].
Stereology has advantages inherent to the method as turning the results into numeric values, with easy reproducibility and comparison between groups and, most importantly, presents a well define theoretic basis.
The equation was used to calculate the volume density of the collagen fibers, where: Vv = volumetric density, Ps = the number of structure points studied (collagen), and, Pp = the number of possible test points (42 in this case).
Levels of testosterone were evaluated using the radioimmunoassay kit DSL-4100 Testosterone®, manufactured by Diagnostics Systems Laboratories Inc. (Webster, TX, USA) and imported by Genesis Diagnostics Products Ltd. (São Paulo, Brazil). All samples were analyzed in duplicate with theoretical sensitivity or 0.05 ng/ml minimum detection limit.
The data obtained were analyzed using the SigmaPlot 12.0 software. The results were validated by analysis of variance (multivariate analysis). Pearson’s Regression, non-parametrical t test and the nonparametric Kruskal–Wallis test were used to assess the differences between the independent samples. The significance criterion used was two-sided p < 0.05.
Results
The mean body weights of rats between the two groups throughout the duration of the experiment were compared using the Kruskal–Wallis test at 95% of probability, showing that there was no change in body weight between the groups ().
Table 1. Average mice body weight in grams over time and groups involved in the experiment.
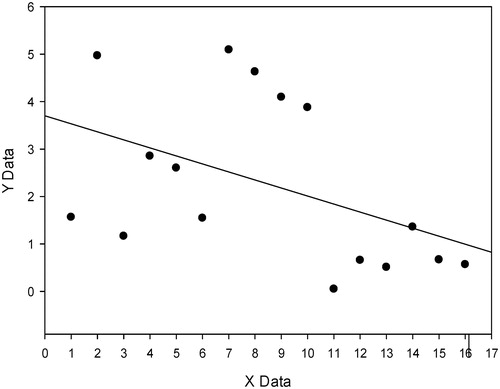
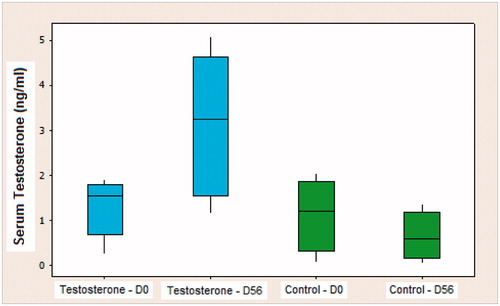
The mean serum testosterone of the testosterone group on the first day (D0) of experiment was 1.196 ng/ml. After hormone replacement therapy, this index reached a value of 3.240 ng/ml, measured on the 56th day (D56). On the other hand, the control group had an average of 1.173 ng/ml on D0, which decreased to 0.635 ng/ml of serum testosterone at the end of the study (D56). According to the analysis of variance (ANOVA), by the Tukey test, the testosterone group replacement therapy was effective and satisfactory, while the androgenic decline continued by the time of experiment in the control group. This showed that there was a significant hormone deficit at the time of sacrificing the control animals (p < 0.05) ().
Figure 1. Box-plot of the values of serum testosterone in the testosterone and control groups on D0 (beginning of the experiment) and D56 (animal sacrifice).
presents the descriptive analysis of the volumetric density of the collagen fibers in the penile cavernous corpora muscle in both groups of the study. It was observed that the testosterone group had a volumetric density of collagen of 20.6%, which was lower than control group (47.8%) showing statistical significance, t test (p < 0.001 and r2 = 0.771) ().
Figure 2. Box-plot of volumetric density of the collagen fibers of the bladder wall in different groups.
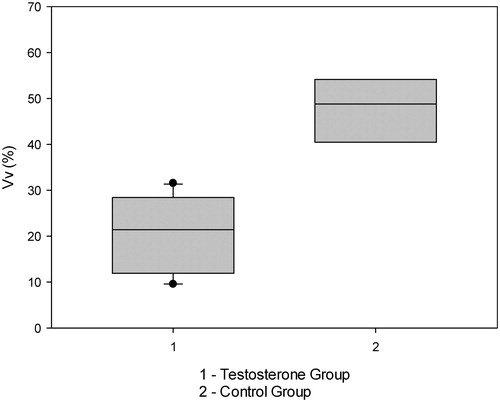
Table 2. Descriptive analysis on collagen fibers of bladder wall in both groups.
The linear regression, with Pearson’s correlation demonstrated an inverse and statistical significant correlation between the density of collagens fiber and the level of testosterone in both groups (p < 0.001 and r2 = 0.538) ().
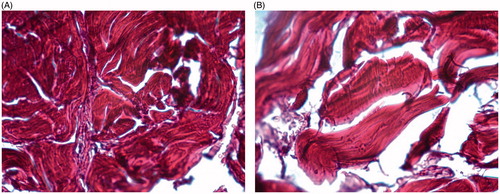
shows picrosirius-stained cavernous corpora highlighting collagen fibers distributed among muscle fibers, ranging mainly from an orange to intense red. In the testosterone group, the presence of less collagen fibers between the detrusor is observed when compared to the control group.
Discussion
The presence of androgen receptor in rat penile and the modulation of autonomic pelvic plexus by testosterone [Citation25] indirectly reinforce the influence of androgenic hormone in the lower genital and urinary tract.
Takyu [Citation26] observed that castration decreased the function of α1-adrenergic and muscarinic receptors, restoring their functions after testosterone replacement. Moreover, the existence of angiotensin II (AII) receptors in the bladder, especially type 2, are related to inflammatory and apoptotic stimuli. Nakazawa et al. [Citation17], using castrated rats, observed increased expression of caspase-3 in the bladder mediated by type 2 AII receptor, when compared to control and testosterone replacement groups. Fraga-Silva et al. [Citation18] found out similar processes on penile structure, mediated by angiotensin receptors system, causing penile fibrosis.
Fillipi et al. [Citation27] found that the expression of phosphodiesterase-5 (PDE 5) in the bladder – an important enzyme that inhibits the NO cycle – is dependent on the levels of circulating androgens and Sánches et al. [Citation21] demonstrated the effects of NO signaling over the oxidative stress process in penis. Furthermore, the RhoA/Rho-kinase system has been investigated for years as a cause of urinary tract disorders, including participation in the overactive bladder, due to its activation of actin–myosin complex that causes muscle contractions, regardless of the levels of cytosolic free calcium. This pathway is also modulated by sex hormones and the ratio estrogen/testosterone imbalance, as occurs in aging and obesity [Citation3], which seems to be the most important factor in the stimulation of RhoA/Rho-kinase [Citation28].
Bhasin et al. [Citation29] and Singh et al. [Citation30,Citation31] believe that androgens stimulate concomitantly the pluripotent cells to differentiate into muscle lineages including smooth muscle, inhibiting strains for the differentiation of adipocytes. To support this finding, Lorenzetti [Citation13], in assessing the influence of testosterone on the bladder wall fibrosis in rats, observed that the sudden drop of testosterone by castration of young rats caused more muscle replacement by collagen fibers when compared to senile rats, whose process of hormonal decline is slow and gradual. Although the orchiectomy model is usually used to assess the influence of testosterone on the bladder, it does not seem appropriate to reproduce the process of late onset hypogonadis. Therefore, we decided to use only senile animals (18–28 months) in this work, which represents more risks and higher costs, but portrays adequately the physiological aging process, avoiding the sample selection bias.
Baseline levels of testosterone in both groups were below the levels described by Kinoshita et al. [Citation32] in 7-month-old rats (2.2 ± 0.3 ng/dl average testosterone), but very similar to the 21-month-old rats (1.1 ± 0.2 ng/dl average testosterone). This fact demonstrates the physiologic decrease of testosterone in rats in this study. Early studies using the replacement model with 50 mg/kg of TU per month have been conducted for about 12 years, showing that this choice is simple, reproducible and effective for hormone replacement in rats [Citation33,Citation34]. Another variation of the experimental model of hormone supplementation, described more recently, uses 100 mg/kg of TU, in a single dose, and its effects can be evaluated after 2 months of administration [Citation35]. Monthly dosage was chosen in this study for its consolidation in literature. This method of supplementation caused a mean serum testosterone of about 3.2 ng/dl in the group that underwent hormone replacement therapy, which was not too superior as the values of young rats (2.2 ± 0.3 ng/dl average testosterone) [Citation32].
For quantitative analysis of fibrotic reaction in the penile cavernous muscle, it was performed the stereological study of collagen fibers in both groups [Citation24]. The volume density (Vv), magnitude used in this study is a stereological parameter that produces reliable results with minimal variation. At the same time, it is not dependent on histologic complex blade or experience of the researcher, being employed in the quantification of fibrous component of the extracellular matrix, particularly in ace collagen and elastic fibers of various tissues [Citation36–38].
Under polarized light, type I collagen fibers appear as being thick, strongly birefringent, with colors ranging from yellow to intense red [Citation39]. The use of polarized light for analysis of picrosirius red stained samples is a special procedure for histological analysis of type-specific collagen [Citation40], and it is not necessary to evaluate fibrotic processes, especially due to the prevalence of type I collagen in these processes. Nevertheless, picrosirius red polarization facilitates the identification and counting of collagen mass in relation to the detrusor muscle, improving the accuracy of the method [Citation39]. The time between intervention and evaluation of the final result on this issue was 2 months and this period of time has been the most used to assess changes in rat muscles [Citation35,Citation41,Citation42].
Ludwig et al. [Citation42] found oxidative stress and apoptosis increased in the bladder of rats subjected to castration; however, it could be reduced by supplementation with α-tocopherol. Still, it was not clear whether the rise of the apoptotic process increases the risk of bladder dysfunction. In fact, oxidative stress is one of the most important factors involved in the pathogenesis of age-related detrusor dyskinesia [Citation43] and may be aggravated by diabetes mellitus [Citation44] and by the hypogonadism [Citation42]. On the other hand, vitamin E produces protective effect against free radicals, especially when used in an early stage of tissue injury [Citation46]. Helmy and Senbel [Citation45] proved that antioxidant therapy with vitamin E ameliorates the age-associated erectile dysfunction and Zhang et al. correlated an antioxidant dietary with the improvement of arteriogenic erectile dysfunction [Citation46].
Cayan et al. [Citation47] showed that rats with bilateral orchiectomy, testosterone replacement associated with estradiol had better results than androgen replacement alone regarding the preservation of the muscle/collagen ratio, demonstrating the stroma modulating role of testosterone, regardless of gender [Citation47]. Tek et al. [Citation35] used the model of castrated 10-month-old rats to evaluate the effect of testosterone replacement (100 mg/kg TU dose) on bladder function and histology. They concluded that hormone replacement in these animals produced an improvement in the smooth muscle/collagen ratio, including the development of bladder capacity.
Our study was a pioneer in demonstrating that testosterone, even lower than the doses used in the works mentioned above, favored the gradual development of smooth muscle cells in penis of rats with senile hormonal decline and testosterone supplementation in aged rats protected them against the process of remodeling/fibrosis of the penis. These finding can implicate that the testosterone levels can influence, not only the libido, but also the erectile function in elderly population.
Declaration of interest
The authors report no conflict of interests.
References
- Coyne KS, Kaplan SA, Chapple CR, et al. Risk factors and comorbid conditions associated with lower urinary tract symptoms: EpiLUTS. BJU Int 2009;103:24–32
- Abdo CHN, Oliveira WM Jr, Scanavino MT, Martins FG. Disfunção erétil – resutlados do estudo da vida sexual do brasileiro. Rev Ass Med Brasileira 2006;52:424–9
- Martini AC, Molina RI, Ruiz RD. Fiol de Cuneo M: obesity and male fertility. Rev Fac Cien Med Nac Cordoba 2012;69:102–10
- Masick JM, Levin RM, Hass MA. The effect of partial outlet obstruction on prostaglandin generation in the rabbit urinary bladder. Prostaglandin Other Lipid Mediat 2001;66:211–19
- de Barros CA, Lorenzetti F, Ortiz V, Dambros M. Testosterone supplementation’s effects on age-related bladder remodeling – experimental study in rats. Aging Male 2013;16:102–7
- Wyllie AH, Kerr JF, Currie AR, et al. Cell death: the significance of apoptosis. Int Rev Cytol 1980;68:251–306
- Higami Y, Shimokawa I, et al. Effect of aging and dietary restriction on hepatocyte proliferation and death in male F344 rats. Cell Tissue Res 1997;288:69–77
- Warner HR. Apoptosis: a two-edged sword in aging. Ann N Y Acad Sci 1999;887:1–11
- Higami Y, Shimokawa I. Apoptosis in the aging process. Cell Tissue Res 2000;301:125–32
- Warner HR. Aging and regulation of apoptosis. Curr Top Cell Regul 1997;35:107–2
- Dambros M, Jongh R, Koeveringe G, et al. Galagin protects pig detrusor nerves from repetitive field stimulation and anoxia-glucopenia injuries. Urology 2005;66:1327–31
- Cabelof DC, Raffoul JJ, Ge Y, et al. Age-related loss of the DNA repair response following exposure to oxidative stress. Gerontol A Biol Sci Med Sci 2006;61:427–34
- Lorenzetti F. Influência dos níveis de Testosterona sobre a densidade volumétrica de fibras colágenas e do processo apoptótico na parede vesical de ratos jovens, senis e orquiectomizados. [tese]. São Paulo: Universidade Federal de São Paulo; 2009
- Holmäng S, Mårin P, Lindstedt G, Hedelin H. Effect of long-term oral testosterone undecanoate treatment on prostate volume and serum prostate-specific antigen concentration in eugonadal middle-aged men. Prostate 1993;23:99–106
- Watkins TW, Keast JR. Androgen-sensitive preganglionic neurons innervate the male rat pelvic ganglion. Neuroscience 1999;93:1147–57
- Hall R, Andrews PL, Hoyle CH. Effects of testosterone on neuromuscular transmission in rat isolated urinary bladder. Eur J Pharmacol 2002;449:301–9
- Nakazawa R, Tanaka M, Takahashi T, et al. Effects of castration and testosterone administration on angiotensin II receptor mRNA expression and apoptosis-related proteins in rat urinary bladder. Endocrine J 2007;54:211–19
- Fraga-Silva RA, Savegnini SQ, De Souza FB, et al. An oral formulation od Angiotensine (1-7) reverses cavernous damages induced by hypercholesterolemia. J Sex Med 2013;10:2430–42
- Kilarkaje N, Yousif MH, El-Hashim AZ, et al. Role of angiotensin-(1-7) in diabetes induced oxidative DNA damage in the corpus cavernosum. Fertil Steril 2013;100:226–33
- Pradidarcheep W. Lower urinary tract symptoms and its potential relation with late-onset hypogonadism. Aging Male 2008;11:51–5
- Sánches A, Contreras C, Martinez MP, et al. Role of neural NO synthase (nNOS) uncoupling in the dysfunctional nitregic vasorelaxation of penile arteries from insulin-resistant obese Zucker rats. PLoS One 2012;7:e36027
- van Herck H, Baumans V, Brandt CJWM, et al. Blood sampling from the retro-orbital plexus, the saphenous vein and the tail vein in rats: comparative effects on selected behavioural and blood variables. Lab Anim 2001;35:131–9
- Dambros M. Influência da ooforectomia bilateral no tecido conjuntivo vesical de ratas. [tesis]. Campinas: Universidade Estadual de Campinas; 2001
- Mandarim-de-Lacerda CA. Stereological tools in biomedical research. Anais da Academia Brasileira de Ciências 2003;75:469–86
- Keast JR. The autonomic nerve supply of male sex organs – an important target of circulating androgens. Behaviour Brain Res 1999;105:81–92
- Takyu S. Effects of testosterone on the autonomic receptor-mediated function in lower urinary tract from male rabbits. Nippon Hinyokika Gakkai Zasshi 1993;84:330-8
- Filippi S, Morelli A, Sandner P, et al. Characterization and functional role of androgen- dependent PDE- 5 activity in the bladder. Endocrinology 2007;148:1019–29
- Chavalmane AK, Comeglio P, Morelli A, et al. Sex steroid receptors in male human bladder: expression and biological function. J Sex Med 2010;7:2698--713
- Bhasin S, Taylor WE, Singh R, et al. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol 2003;58A:1103–10
- Singh R, Artaza JN, Taylor WE, et al. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor- mediated pathway. Endocrinology 2003;144:5081–8
- Singh R, Artaza JN, Taylor WE, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with betacatenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology 2006;147:141–54
- Kinoshita Y, Higashi Y, Winters S, et al. An analysis of the age-related decline in testicular steroidogenesis in the rat. Biol Reprod 1985;32:309–14
- Shen Z, Chen Z, Lu Y, et al. Relationship between gene expression of nitric oxide synthase and androgens in rat corpus cavernosum. Chin Med J (Engl) 2000;113:1092–5
- Shen ZJ, Lu YL, Chen ZD, et al. Effects of androgen and ageing on gene expression of vasoactive intestinal polypeptide in rat corpus cavernosum. BJU Int 2000;86:133–7
- Tek M, Balli E, Çimen B, et al. The effect of testosterone replacement therapy on bladder functions and histology in orchiectomized mature male rats. Urology 2010;75:886–90
- Carvalho Junior AM. Estereologia do Sistema Elástico e Distribuição da Laminina na Zona de Transição de Próstatas Normais e com Hiperplasia Benigna. Dissertação. Rio de Janeiro. Universidade do Estado do Rio de Janeiro; 2002
- Costa WS, Carvalho AM, Babinski MA, et al. Volumetric density of elastic and reticular fibers in transition zone of controls and patients with benign prostatic hyperplasia. Urology 2004;64:693–7
- Dambros M, Rodrigues Palma PC, Mandarim-de-Lacerda CR, et al. The effect of ovariectomy and estradiol replacement on collagen and elastic fibers in the bladder of rats. Int Urogynecol J 2003;14:108–12
- Montes GS, Junqueira LC. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz 1991;86:1–11
- Montes GS. Structural biology of the fibers of the collagenous and elastic systems. Cell Biol Int 1996;20:15–27
- Nnodim JO. Quantitative study of the effects of denervation and castration on the levator ani muscle of the rat. Anat Rec 1999;255:324–33
- Ludwig L, Seraphim D, Lorenzetti F, et al. Inhibitory activity of a-tocoferol on apoptosis in the rat bladder wall subjected to androgen deprivation. Neurourol Urodynamics 2011;30:194–8
- Gómez-Pinilla PJ, Pozo MJ, Camello PJ. Aging impairs neurogenic contraction in guinea pig urinary bladder: role of oxidative stress and melatonin. Am J Physiol Regul Integr Comp Physiol 2007;293:R793–803
- Aybek H, Aybek Z, Abban G, Rota S. Preventive effects of vitamin E against oxidative damage in aged diabetic rat bladders. Urology 2011;77:508.e10–4
- Helmy MM, Senbel AM. Evaluation of vitamin E in treatment of erectile dysfunction in aged rats. Life Sci 2012;9,90(13–14):489–94
- Zhang Q, Radisavljevic ZM, Siroky MB, Azadzoi KM. Dietary antioxidants improve arteriogenic erectile dysfunction. Int J Androl 2011;34:225–35
- Çayan F, Tek M, Balli E, et al. The effect of testosterone alone and testosterone + estradiol therapy on bladder functions and smooth muscle/collagen content in surgically menopause induced rats. Maturitas 2008;60:248–52