Abstract
Late-onset hypogonadism (LOH) and depression contribute to cardiovascular disease (CVD) in male hemodialysis (HD) patients. Carnitine deficiency is frequently observed in HD patients, playing a role in CVD. We examined whether carnitine deficiency was independently associated with LOH and depression in these patients. Twenty-six male HD patients underwent determinations of serum levels of free carnitine and testosterone. Status of LOH and depression were evaluated by questionnaires using aging male symptoms’ (AMS) scale and self-rating depression scale (SDS), respectively. Free carnitine and testosterone levels in male HD patients were significantly lower than those in age-matched healthy male subjects. Linear regression analysis showed that AMS scale was positively associated with SDS. Univariate regression analysis revealed that total carnitine (inversely), free carnitine (inversely) and HD duration were correlated with AMS scale. Multiple stepwise regression analysis revealed that free carnitine was an independent determinant of AMS scale. Furthermore, free carnitine was also independently correlated with SDS in male HD patients. This study demonstrated that decreased free carnitine levels were independently associated with AMS scale and SDS in male HD patients. The observations suggest that decreased free carnitine levels could be a marker and therapeutic target of LOH and depression in uremic men with HD.
Introduction
Late-onset hypogonadism (LOH) has been linked to poor quality of life and increased mortality in patients with end-stage renal disease [Citation1]. About 52% of male hemodialysis (HD) patients were diagnosed as hypogonadism on the basis of low concentration of serum testosterone levels [Citation2]. Furthermore, low testosterone levels have also been associated with the risk of cardiovascular disease (CVD) and all-cause mortality in male patients on HD [Citation2,Citation3]. On the other hand, depression, a mental disorder with a high personal, societal and economic impact, affects at least 20–30% of patients receiving HD therapy [Citation4]. Depression could decrease the adherence to medication and perceived quality of life in patients undergoing HD [Citation5,Citation6]. In addition, it is not only associated with suicide risk but also correlated with the increased risk for CVD in patients on HD as well [Citation7,Citation8]. Although several factors such as malnutrition, inflammation, erythropoietin resistance, environmental stress and atherosclerosis have been proposed to contribute to the progression of LOH and depression in uremic men [Citation9–12], the precise underlying mechanism is not fully understood. Moreover, therapeutic options for LOH and depression in male HD subjects are far from satisfactory [Citation5,Citation13,Citation14]. Therefore, to identify a novel therapeutic target that could ameliorate LOH and depression is urgently needed for improving quality of life and preventing the progression of CVD in male HD patients.
Carnitine is a natural substance, which is supplied by the intake of protein-rich foods and also synthesized by the liver, kidney, skeletal, cardiac muscles, brain, epididymis and testis in humans [Citation15,Citation16]. Carnitine is involved in fatty acid β-oxidation and energy production by transporting long-chain fatty acids from the cytoplasm to mitochondria [Citation16]. We, along with others, have reported that serum carnitine levels are significantly decreased in HD patients [Citation17,Citation18]. Since carnitine supplementation has been shown to improve aging-related sexual dysfunction assessed by nocturnal penile tumescence and International Index of Erectile Function Score and ameliorate cancer-related depression [Citation19,Citation20], carnitine deficiency may be an important causative factor for the progression of LOH and depression in male HD patients. However, it remains unclear which anthropometric, metabolic, clinical and biochemical variables, including serum carnitine, are independently correlated with LOH and depression in male patients with HD. Therefore, in this study, we examined (1) the correlation between LOH and depression status assessed by aging male symptoms’ (AMS) scale and self-rating depression scale (SDS), respectively, and (2) which anthropometric valuables were independently associated with AMS scale and SDS in male HD subjects.
Methods
Patients
Twenty-six male patients receiving chronic HD (mean age: 63.4 ± 10.0 years; mean duration of HD: 51.3 (10–185) months) and age-matched 15 healthy male subjects (mean age: 57.6 ± 7.1 years) underwent a complete history, physical examination and determinations of blood chemistries, including serum total, free and acylcarnitine and free testosterone. Patients were dialyzed for 4–5 h with high-flux dialyzers three times a week. Ten patients had diabetes mellitus, 23 patients received inhibitors of renin–angiotensin system (RAS) and five statins for the treatment of dyslipidemia.
Informed consent was obtained from all patients, and the study protocol was approved by the Institutional Ethics Committees of Kurume University School of Medicine, Japan.
Data collection
The medical history was ascertained by a questionnaire. Blood pressure was measured in the sitting position using an upright standard sphygmomanometer just before starting HD. Vigorous physical activity and smoking were avoided for at least 30 min before blood pressure measurement.
General symptoms for LOH were judged according to the AMS scale in all male patients [Citation21]. Nineteen patients were further examined depression status by SDS [Citation22]. The AMS scale is designed as self-administrated scale to assess symptoms of aging which consists of general well-being, joint pain and muscular ache, excessive sweating, sleep problems, increased need for sleep and often feeling tired, irritability, nervousness, anxiety, physical exhaustion, decrease in muscular strength, depressive mood, feeling burnt out, decrease in beard growth, decrease in ability/frequency to perform sexually, morning erections and sexual desire/libido [Citation21]. Each question is scored on a scale of one through five. The SDS is designed to assess the level of depression for patients diagnosed with depressive disorder [Citation22]. The SDS is a short self-administered survey to quantify the depressed status of a patient. There are 20 items on the scale that rate affective, psychological and somatic symptoms associated with depression. There are 10 positively worded and 10 negatively worded questions. Each question is scored on a scale of one through four.
Blood was drawn from arteriovenous shunt just before starting HD sessions for the determinations of hemoglobin, total protein, lipids (low-density lipoprotein-cholesterol and triglycerides), blood urea nitrogen, creatinine, uric acid, calcium and phosphate. Intact parathyroid hormone (PTH) was evaluated by an immunoradiometric assay (Allegro I-PTH; Nichols Institute, San Juan Capistrano, CA). β2-microglobulin was measured by a latex immunoagglutination assay (Eiken Chemical Co., Ltd. Tokyo, Japan). Serum carnitine levels were determined as described previously [Citation23]. Free testosterone levels were measured by electro-chemiluminescence immunoassay and radioimmunoassay, respectively (SRL Inc., Tokyo, Japan). Other blood chemistries were measured at a commercially available laboratory (Wako Pure Chemical Industries, Ltd, Osaka, Japan) as described previously [Citation24]. Efficacy of HD was also evaluated by a single-pool fractional clearance of body water for urea (Kt/V) as described previously [Citation25].
Statistical analysis
Data are presented as mean ± standard deviation. Use of RAS inhibitors and statins and the presence or absence of diabetes mellitus were coded as dummy variables. Since HD duration, triglycerides and intact PTH levels were not normally distributed, log-transformed values were used for statistical analysis. To compare clinical values between healthy controls and HD patients, unpaired t-test was performed. To evaluate the correlation between free carnitine and AMS scale and SDS or AMS scale and SDS, univariate regression analysis was performed. To determine the independent correlates of AMS scale and SDS, multiple stepwise regression analysis was performed. Statistical significance was defined as p < 0.05. All statistical analyses were performed with SPSS version 20 (Chicago, IL) system.
Results
Demographic data
Demographic data are shown in . Total and free carnitine and free testosterone levels in male HD patients were significantly lower, whereas acylcarnitine levels and acyl-to-free carnitine ratio were significantly higher than those in age-matched healthy subjects (total carnitine: 43.1 ± 10.4 versus 59.0 ± 6.0 µmol/l, p < 0.001, free carnitine: 25.2 ± 5.7 versus 48.1 ± 5.7 µmol/l, p < 0.001, free testosterone: 6.06 ± 2.48 versus 10.13 ± 3.54 pg/ml, p < 0.001, acylcarnitine: 17.9 ± 5.7 versus 15.4 ± 5.8 µmol/l, p < 0.001, acyl/free carnitine ratio: 0.72 ± 0.18 versus 0.23 ± 0.05, p < 0.001). AMS scale and SDS in HD patients were higher than normal reference ranges (36.8 ± 11.8, 43.9 ± 10.2, respectively, normal reference ranges: AMS scale <27, SDS: 20–44).
Table 1. Clinical characteristics of the patients.
Correlates of AMS scale
Univariate regression analysis showed that AMS scale was positively associated with SDS in HD patients (r2 = 0.366, p = 0.006; ). Furthermore, HD duration (p = 0.048), total carnitine (inversely, p = 0.013) and free carnitine (inversely, p = 0.003, ) were significantly associated with AMS scale (). Because these significant parameters could be closely correlated with each other, multiple regression analysis was performed. Multiple stepwise regression analysis showed that free carnitine (β = −0.559, p = 0.003) was a sole independent correlate of AMS scale (r2 = 0.313; ).
Figure 1. Correlation between AMS scale and SDS in male HD patients (n = 19). AMS, aging male symptom; SDS, self-rating depression scale; and HD, hemodialysis.
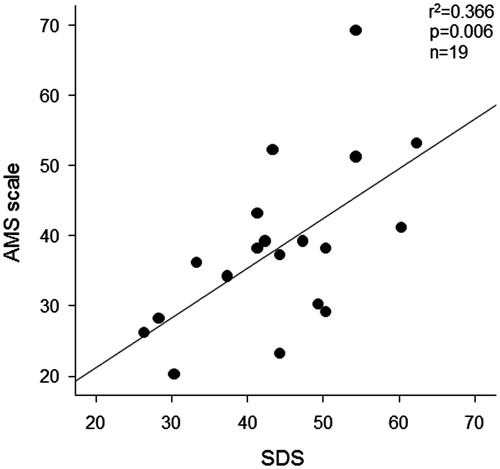
Figure 2. (A) Correlation between AMS scale and free carnitine levels in male HD patients (n = 26). (B) Correlation between SDS and free carnitine levels in male HD patients (n = 19). AMS, aging male symptom; SDS, self-rating depression scale; and HD, hemodialysis.
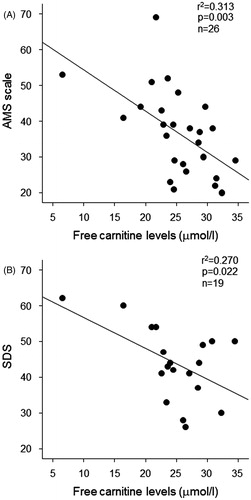
Table 2. Univariate and multiple stepwise regression analysis for the correlates of AMS scale.
Correlates of SDS
We next examined which clinical variables are independent determinants of SDS in our subjects. Univariate regression analysis revealed that intact PTH (p = 0.028) and free carnitine (inversely, p = 0.022; ) were correlated with SDS in HD patients (). In the multiple stepwise regression analysis, free carnitine was found to be independently associated with SDS (r2 = 0.270; ).
Table 3. Univariate and multiple stepwise regression analysis for the correlates of SDS.
Discussion
We demonstrated In this study that (1) serum free testosterone and free carnitine levels were significantly decreased and that AMS scale and SDS were higher in men with HD; (2) there was a positive correlation between AMS scale and SDS in HD patients; and (3) free carnitine was a sole independent determinant of AMS scale and SDS in male subjects on HD.
There are a couple of papers to suggest the correlation between LOH and depression status in aging male [Citation26,Citation27]. Patients with LOH had significantly higher scores for Beck Depression Inventory and Beck Anxiety Inventory [Citation26]. Testosterone replacement therapy has been reported to not only improve the low testosterone-related symptoms but also ameliorate depression subscale in LOH patients in Chinese population [Citation27]. Furthermore, depression score was found an independent risk factor for the development of erectile dysfunction in HD patients [Citation28]. In this study, since free carnitine, but not testosterone levels, were an independent determinant of both AMS scale and SDS, carnitine deficiency rather than decreased free testosterone values may be a common soil for the progression of LOH and depression in male HD patients. These observations suggest that L-carnitine administration may be a novel therapeutic target for preventing the progression of LOH and depression mode in male uremic patients with HD.
In this study, total and free carnitine levels were negatively associated with AMS scale in HD patients. Carnitine is expressed in the epididymis and testis in humans and its levels in the seminal plasma were associated with sperm motility [Citation15]. Furthermore, carnitine supplementation has been shown to ameliorate aging-related sexual dysfunction in aged men [Citation19] and augment the efficacy of sildenafil, an inhibitor of phosphodiesterase-5, which could restore the sexual potency after bilateral nerve-sparing radical retropubic prostatectomy [Citation29]. In addition, supplementation of carnitine has been reported to inhibit the development and progression of CVD in animal models [Citation30], free fatty acid-induced endothelial dysfunction [Citation31] and decline in mental performances [Citation32] in humans. Therefore, carnitine supplementation might also prevent the progression of LOH and subsequently reduce the risk of CVD in male HD patients.
We did not know in this study the exact mechanisms for the correlation between low free carnitine levels and depression in male HD subjects. However, L-carnitine supplementation significantly improved depression mode and fatigue in carnitine-deficient patients with cancer [Citation20]. Moreover, intensive L-acetylcarnitine treatment has been reported to ameliorate main mental parameters of the senile brain in humans [Citation33]. Since depression status could also be closely correlated with LOH, carnitine supplementation may act on central nerve system by partly improving the gonadal function, which could lead to the amelioration of depression mode in male HD patients.
Limitations
There are several limitations in this study. First, sample size of this study was small. Second, it was a cross-sectional study, and therefore, could not assess the question whether deficiency of serum carnitine levels was a cause or consequence of LOH syndrome or depression. In uremic patients with HD, more than 80% of free carnitine was eliminated from the blood via HD [Citation18]. So, our observations suggest that free carnitine levels could be a marker of LOH and depression status in male HD patients. However, it remains unclear whether carnitine could also be a marker and therapeutic target in female HD patients. Further longitudinal and/or interventional studies are needed to clarify whether carnitine supplementation could improve LOH and depression mode and subsequently reduce the risk of future CVD in male patients with HD.
Declaration of interest
The authors have no conflicts of interest to declare.
This work was supported, in part, by a Grant-in-Aid for Welfare and Scientific Research (C) (no. 25461239) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (K.F) and by Grants of MEXT-Supported Program for the Strategic Research Foundation at Private Universities, the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (S. Y.).
References
- Carrero JJ, Stenvinkel P. The vulnerable man: impact of testosterone deficiency on the uraemic phenotype. Nephrol Dial Transplant 2012;27:4030–41
- Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol 2009;20:613–20
- Kyriazis J, Tzanakis I, Stylianou K, et al. Low serum testosterone, arterial stiffness and mortality in male haemodialysis patients. Nephrol Dial Transplant 2011;26:2971–7
- Agganis BT, Weiner DE, Giang LM, et al. Depression and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis 2010;56:704–12
- Cukor D, Rosenthal DS, Jindal RM, et al. Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int 2009;75:1223–9
- Park HC, Yoon HB, Son MJ, et al. Depression and health-related quality of life in maintenance hemodialysis patients. Clin Nephrol 2010;73:374–80
- Martiny C, de Oliveira e Silva AC, Neto JP, Nardi AE. Factors associated with risk of suicide in patients with hemodialysis. Compr Psychiatry 2011;52:465–8
- Boulware LE, Liu Y, Fink NE, et al. Temporal relation among depression symptoms, cardiovascular disease events, and mortality in end-stage renal disease: contribution of reverse causality. Clin J Am Soc Nephrol 2006;1:496–504
- Cano NJ, Heng AE, Pison C. Multimodal approach to malnutrition in malnourished maintenance hemodialysis patients. J Ren Nutr 2011;21:23–6
- Iglesias P, Carrero JJ, Díez JJ. Gonadal dysfunction in men with chronic kidney disease: clinical features, prognostic implications and therapeutic options. J Nephrol 2012;25:31–42
- Fahed AC, Gholmieh JM, Azar ST. Connecting the lines between hypogonadism and atherosclerosis. Int J Endocrinol 2012;2012:793–953
- Afsar B. The relationship between depressive symptoms and erythropoietin resistance in stable hemodialysis patients with adequate iron stores. Int J Artif Organs 2013;36:314–9
- Chatterjee R, Wood S, McGarrigle HH, et al. A novel therapy with testosterone and sildenafil for erectile dysfunction in patients on renal dialysis or after renal transplantation. J Fam Plann Reprod Health Care 2004;30:88–90
- Francomano D, Bruzziches R, Natali M, et al. Cardiovascular effect of testosterone replacement therapy in aging male. Acta Biomed 2010;81:101–6
- Bøhmer T, Hoel P, Purvis K, Hansson V. Carnitine levels in human accessory sex organs. Arch Androl 1978;1:53–9
- Evans AM, Fornasini G. Pharmacokinetics of L-carnitine. Clin Pharmacokinet 2003;42:941–67
- Evans A. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am J Kidney Dis 2003;41:S13–26
- Adachi T, Fukami K, Yamagishi S, et al. Decreased serum carnitine is independently correlated with increased tissue accumulation levels of advanced glycation end products in haemodialysis patients. Nephrology (Carlton) 2012;17:689–94
- Cavallini G, Caracciolo S, Vitali G, et al. Carnitine versus androgen administration in the treatment of sexual dysfunction, depressed mood, and fatigue associated with male aging. Urology 2004;63:641–6
- Cruciani RA, Dvorkin E, Homel P, et al. Safety, tolerability and symptom outcomes associated with L-carnitine supplementation in patients with cancer, fatigue, and carnitine deficiency: a phase I/II study. J Pain Symptom Manage 2006;32:551–9
- Heinemann LA, Saad F, Zimmermann T, et al. The Aging Males’ Symptoms (AMS) scale: update and compilation of international versions. Health Qual Life Outcomes 2003;1:15
- Zung WW. A self-rating depression scale. Arch Gen Psychiatry 1965;12:63–70
- Takahashi M, Ueda S, Misaki H, et al. Carnitine determination by an enzymatic cycling method with carnitine dehydrogenase. Clin Chem 1994;40:817–21
- Nagano M, Fukami K, Yamagishi S, et al. Tissue level of advanced glycation end products is an independent determinant of high-sensitivity C-reactive protein levels in haemodialysis patients. Nephrology (Carlton) 2011;16:299–303
- Daugirdas JT. Linear estimates of variable-volume, single-pool Kt/V: an analysis of error. Am J Kidney Dis 1993;22:267–70
- Aydogan U, Aydogdu A, Akbulut H, et al. Increased frequency of anxiety, depression, quality of life and sexual life in young hypogonadotropic hypogonadal males and impacts of testosterone replacement therapy on these conditions. Endocr J 2012;59:1099–105
- Zhang XW, Liu ZH, Hu XW, et al. Androgen replacement therapy improves psychological distress and health-related quality of life in late onset hypogonadism patients in Chinese population. Chin Med J (Engl) 2012;125:3806–10
- Fernandes GV, dos Santos RR, Soares W, et al. The impact of erectile dysfunction on the quality of life of men undergoing hemodialysis and its association with depression. J Sex Med 2010;7:4003–10
- Cavallini G, Modenini F, Vitali G, Koverech A. Acetyl-L-carnitine plus propionyl-L-carnitine improve efficacy of sildenafil in treatment of erectile dysfunction after bilateral nerve-sparing radical retropubic prostatectomy. Urology 2005;66:1080–5
- Sayed-Ahmed MM, Khattab MM, Gad MZ, Mostafa N. L-carnitine prevents the progression of atherosclerotic lesions in hypercholesterolaemic rabbits. Pharmacol Res 2001;44:235–42
- Shankar SS, Mirzamohammadi B, Walsh JP, Steinberg HO. L-carnitine may attenuate free fatty acid-induced endothelial dysfunction. Ann N Y Acad Sci 2004;1033:189–97
- Salvioli G, Neri M. L-acetylcarnitine treatment of mental decline in the elderly. Drugs Exp Clin Res 1994;20:169–76
- Bonavita E. Study of the efficacy and tolerability of L-acetylcarnitine therapy in the senile brain. Int J Clin Pharmacol Ther Toxicol 1986;24:511–6
