Abstract
Objective: At present, calculated free testosterone assessment is considered as the gold standard in diagnosing male hypogonadism. However, this assessment is not available for all the individuals diagnosed with decreased testicular function. The investigators of this study were, thus, prompted to evaluate whether the androgen deficiency in the aging male (ADAM) and the Massachusetts Male Ageing Study (MMAS) questionnaires could be used to replace biochemical parameters in the diagnosis for hypogonadism in men aged 40 years and above.
Methods: We evaluated 460 men, aged 40 years and above, all volunteers of a screening program for prostate cancer based at the Hospital de Clínicas of Porto Alegre. In this study, we assessed the efficiency of the ADAM and MMAS questionnaires in diagnosing Brazilian men with low levels of total, calculated free and bioavailable testosterone.
Results: The sensitivity of the ADAM questionnaire in diagnosing the calculated free testosterone was 73.6%, whereas specificity was 31.9%. ADAM could be used to properly classify our cohort into normal or hypogonadal individuals in 52.75% of the cases. The sensitivity of the MMAS questionnaire was 59.9%, whereas the specificity was 42.9%, resulting in a successful classification of 51.4% of the patients.
Conclusion: The ADAM and MMAS questionnaires showed adequate sensitivity in diagnosing male patients with low levels of free testosterone. However, because of the lack of specificity, these tools cannot replace calculated free testosterone assessments in men aged 40 years and above.
Introduction
In the past century, life expectancy among individuals in developed countries has rapidly increased, thus, drawing attention to the physiologic or pathologic conditions that affect the elderly [Citation1,Citation2].
Gonadal function has been earlier shown to decrease with age. Male aging involves changes in hormone levels, resulting in variable and gradual psychologic and physical consequences. In young men, almost 54% of circulating testosterone is bound to albumin in a non-specific manner (low affinity binding), 44% is specifically bound to sex hormone-binding globulin (SHBG, high affinity binding) and 1–3% is unbound and known as free testosterone (FT). FT and albumin-bound testosterone, which possess biological activity, comprise the bioavailable testosterone (BT) [Citation3].
During the process of aging, the levels of total serum testosterone gradually decrease, whereas SHBG concentrations constantly increase. Consequently, the amount and effects of bioavailable testosterone in tissues decrease. Elderly male patients presenting low testosterone levels might experience a decrease in libido, with or without erectile dysfunction, loss of muscle strength, psychological changes such as depression, and increased risk for osteoporosis. These symptoms are now collectively known as androgen deficiency of the aging male (ADAM). However, these are not specific enough to be considered pathognomonic, making it difficult to distinguish ADAM from natural aging using a simple symptoms assessment [Citation4,Citation5].
The current gold standard in diagnosing primary or secondary hypogonadism involves the assessment of serological free testosterone. However, economic and technical constraints have hindered its application in individuals with suspected ADAM [Citation1,Citation6].
Over the past two decades, several questionnaires have been developed to identify candidates for hormone replacement therapy. Although satisfactory results were generated using these tools, these questionnaires unequivocally could not replace routine laboratory assessments [Citation7].
The aim of our study was to evaluate whether a self-administered questionnaire might replace the free testosterone measurement performed to diagnose ADAM. This study employed the ADAM questionnaire and Massachusetts Male Aging Study (MMAS) or Smith’s screener questionnaire.
Materials and methods
The research study was conducted with prior approval from the Research Ethics Committee of the Hospital de Clínicas of Porto Alegre (HCPA). All participating patients signed a free and informed consent form.
A cross-sectional analysis carried out in 2010 included Brazilian males with ages 40 years and above. The patients were recruited to the study through the HCPA Annual Screening Program for Prostate Cancer. To obtain a power of 80% and a level of significance of p = 0.05, this study employed a cohort of 223 patients.
Patient exclusion criteria included severe chronic diseases that might have interfered with testosterone serological levels (e.g. diabetes and congestive heart failure), alcohol and/or illicit drug abuse, and recent surgeries or diagnoses of prostate cancer.
Patients were invited to complete the ADAM and MMAS questionnaires. The ADAM questionnaire consisted of 10 items describing the most common symptoms of androgen deficiency. The questionnaire was divided into three sections: physical disposition, mood and sexual performance. The questionnaire was considered positive (A+) when the patient provided a “Yes” answer to any question related to sexual performance or at least three positive responses in the other sections. The test is considered negative (A−) in all the other cases. The aim of using the MMAS questionnaire was to identify eight items that show a strong relationship with the risk of developing hypogonadism (in order of importance): (a) diabetes, (b) obesity (divided into three levels according to body mass index [BMI]), (c) age above 60 years, (d) prevalence of headache, (e) smoking, (f) asthma during treatment, (g) dominant personality (especially at work) and (h) hours of sleep each night. The minimum score obtained with this questionnaire could be 0 (zero), whereas the maximum is 15. The results of the MMAS questionnaire were considered positive when the resulting score is at least five points.
After the completion of the questionnaires, the patients were examined and subjected to brief anamnesis and physical assessment for weigh (kg), height (cm), and waist and hip circumference (cm). BMI and waist–hip ratio were calculated as the follows: BMI = weight/height and WHR = abdominal circumference/Hip circumference.
Blood samples were then collected (scheduled from 9:00 to 11:00 AM) to measure total testosterone (TT), SHBG and albumin. All tests were performed at the Laboratory of Clinical Pathology of the Hospital de Clínicas of Porto Alegre. The samples were centrifuged and the sera were isolated for analysis. TT was measured by radioimmunoassay (DSL-4000 Active), SHBG was assessed by electrochemiluminescence (Modular E170, Roche), and albumin was measured using a colorimetric assay (bromocresol green, Modular P, Roche).
Free testosterone was indirectly measured using the formula proposed by Vermeulen and collaborators [Citation1]:
in which N = 1 + KA × [A]; [TT] = total testosterone in mol/l; [SHBG] = sexual hormone binding globulin in mol/l, [A] = serum albumin in mol/l; KSHBG = affinity constant of testosterone for SHBG = 1 × 109 mol/l and Ka = affinity constant of testosterone for albumin = 3.6 × 104 mol/l.
As references values, we used 346 ng/dl (12 nmol/l) for TT and 65 pg/ml (225 pmol/l) for calculated FT, based on recent recommendations. Unfortunately, no consensus on the normal range of calculated bioavailable testosterone has been established to date. Therefore, our reference value was based on a previous report, in the value of 150 ng/dl (5.3 nmol/l) [Citation8,Citation9].
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were assessed for both ADAM and MMAS questionnaires.
Statistical analysis was performed using SPSS version 21 on a Mac OS X platform. Kolmogorov–Smirnov test was performed to certify data normality. Data correlation was carried out using Pearson’s correlation test (r). Age comparison of androgen parameters was performed using one-way ANOVA and Tukey’s post hoc test. Statistical analysis for qualitative variables was carried out using the chi-squared test. Comparisons between age, hormonal levels, BMI, WHR and waist circumference were conducted using the Student’s t-test. A value of p ≤ 0.05 was considered significant in this study.
Results
In total, we analyzed 465 males with ages 40 years and above. Patient age was further classified into four groups: 40–49 years (23.7%), 50–59 years (34.6%), 60–69 years (32.7%) and ≥70 years (8.9%).
The main complaints collected from the study population were classified as follows: dysfunctional voiding/urinary (19.6%; n = 91), sexual (16.1%; n = 75), dysfunctional voiding/urinary and sexual (6.7%; n = 31), other complaints (4.7%; n = 22) and no complaints (52.9%; n = 246).
The androgen parameters and baseline characteristics of the patients are shown in and , respectively.
Table 1. Demographic characteristics of the population.
Table 2. Correlation between age, BMI, waist circumference, WHR and androgen levels.
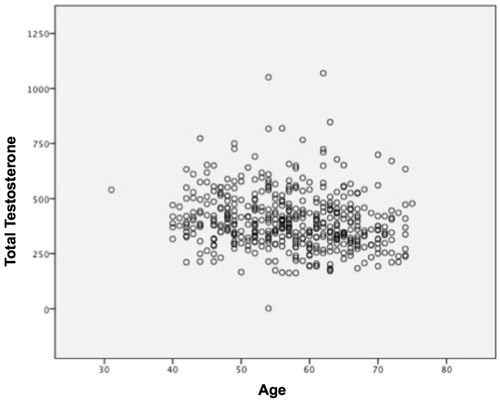
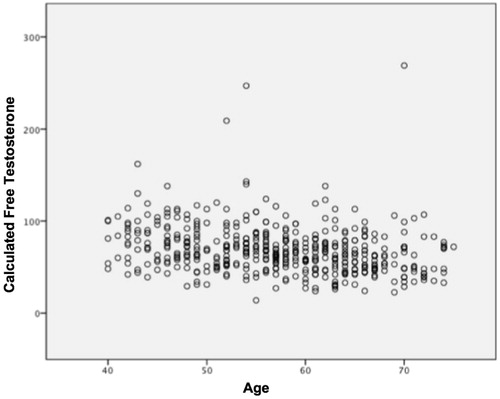
This study showed a negative correlation among patient age, TT (r: −0.102; p = 0.028) () and calculated FT levels (r: −0.151, p < 0.001) (). In addition, TT was shown to have a negative correlation with BMI, whereas waist circumference was observed to have a negative correlation with WHR ().
A significant decrease in the levels of TT, FT and BT during aging was observed. On the other hand, aging led to a gradual increase of SHBG. Out of a total cohort of 459 patients, 223 (48.58%) were classified as hypogonadal according to the FT reference value and 166 (36.16%) according to the TT.
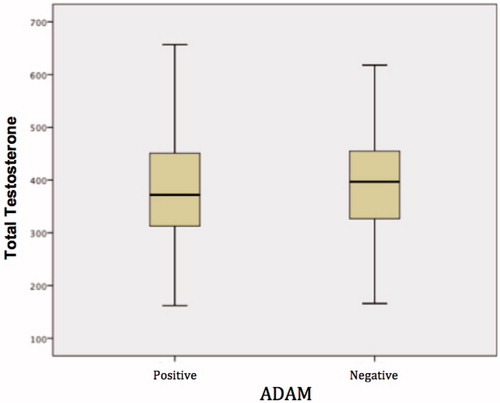
Approximately 70.8% (326/460) of the patients who completed the ADAM questionnaire survey were classified as positive (A+), whereas only 29.2% (134/460) were negative (A−). shows the comparison of androgen levels among patients classified as ADAM positive and ADAM negative. We found a statistically significant difference for calculated FT (p < 0.03) and BT (p < 0.009) ( and ).
Figure 4. Variation in free calculated testosterone levels among patients (ADAM positive and ADAM negative).
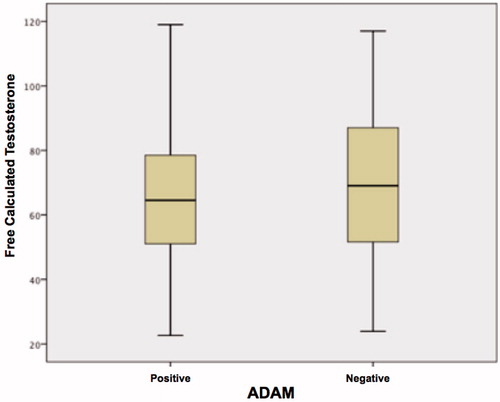
Table 3. Comparison of the androgen levels in patients ADAM positive and ADAM negative.
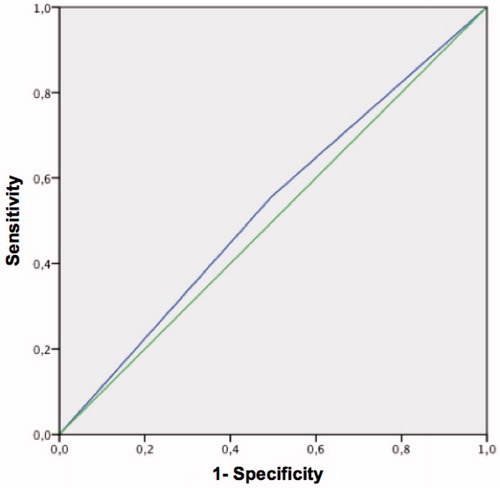
The receiver operating characteristics (ROC) curve from the ADAM questionnaire, for a threshold of 65 pg/ml calculated FT, is shown in . The area under the curve was 0.53 (p < 0.029).
Figure 5. ROC curve of the ADAM questionnaire for free calculated testosterone <65 pg/ml. Area under the curve = 0.53 (p < 0.029).
The results using the MMAS questionnaire were as follows: 43.8% (193/441) of those who answered the questionnaire had a score ≤ 4 (negative MMASq); 51.2% (226/441) were between 5 and 9; 5.0% (22/441) had ≥10.
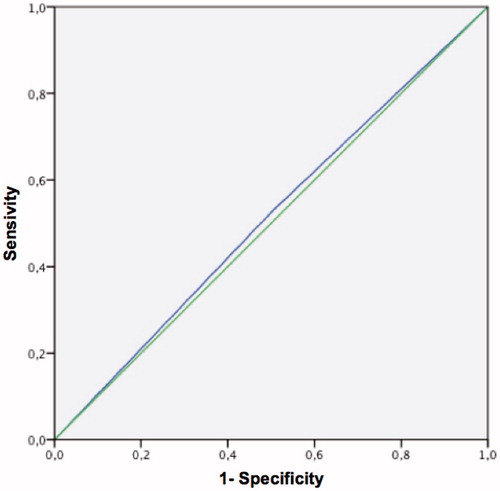
The MMAS ROC curve with a threshold of 65 pg/ml calculated FT is shown in . The area under the curve was 0.513 (p < 0.05).
Figure 6. ROC curve of the MMAS questionnaire for free calculated testosterone <65 pg/ml. Area under the curve = 0.513 (p < 0.05).
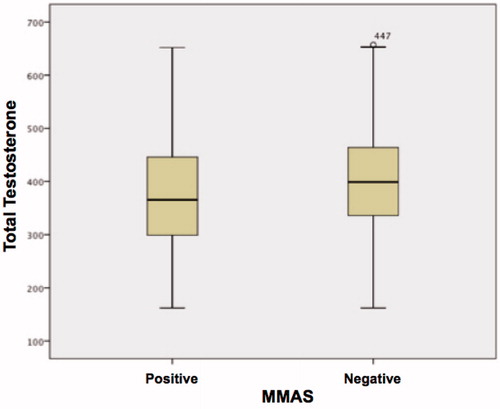
Correlations between average age groups, hormone levels, BMI, WHR and waist circumference, as well as MMAS results (normal score ≤ 4 or abnormal ≥ 5) are presented in . A statistically significant difference was observed in all the items, except for calculated FT (p < 0.067) and SHBG (p < 0.114) ( and ).
Figure 8. Variation in free calculated testosterone levels among patients (MMAS positive and MMAS negative).
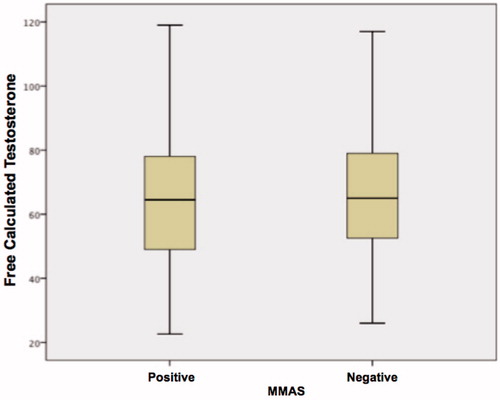
Table 4. Comparison of age, hormone levels, BMI, WHR and waist circumference between patients with normal and anormal score in MMASq.
The sensitivity of the ADAM questionnaire in detecting FT was 76.5%, whereas its specificity was 32.32%. For calculated FT, the sensitivity observed was 73.6%, whereas the specificity was 31.9%; for BT, the sensitivity was 74.7%, whereas the specificity was 31.4% ( and ).
Table 5. Evaluation of the ADAM questionnaire × total testosterone.
Table 6. Evaluation of the ADAM questionnaire × free calculated testosterone.
Using a score of 5 as MMAS cutoff, we detected a sensitivity of 66.4% and a specificity of 46.1% for TT. For calculated FT and BT, the sensitivity was 59.9% and 60.35%, respectively, whereas the specificity was 42.9% in both cases ( and ).
Table 7. Evaluation of the MMAS questionnaire × total testosterone.
Table 8. Evaluation of the MMAS questionnaire × free calculated testosterone.
Discussion
Most of the published reports indicate that hypogonadism should be defined by biochemical criteria and the presence of typical symptoms of testosterone deficiency. The functional criteria are usually assessed by questionnaires, although the sensitivity and specificity of these tests should be individualized for each culture in order to be commonly adopted. Biochemical assessments require measures of total and calculated FT, as previously described.
This study demonstrated a high prevalence of hypogonadism in Brazilian men aged 40 years and older, which should alert us to a disease frequently neglected by medical professionals. Depending on the diagnostic criteria, these indexes can considerably vary. For instance, by using TT as our reference, 166 patients (35.69%) were diagnosed through ADAM, whereas using calculated FT, 223 patients (47.95%) were diagnosed. These results mean that 57 men (or 12.25%) might be classified as presenting normal gonad function, and presumably would not receive proper care. Previous studies also reported that FT might identify a large amount of men suffering from hypogonadism [Citation10–13].
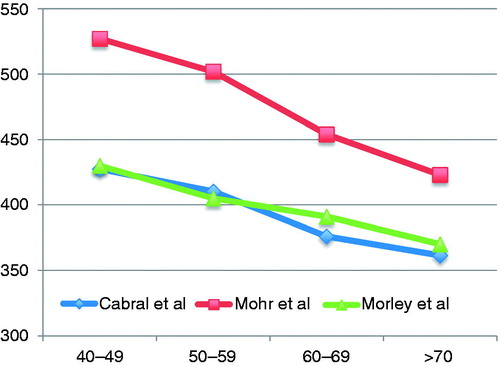
This study showed that age plays a relevant, if not the most important, role in the decrease of TT levels, in agreement with previous published work (). However, we observed that the most significant reduction occurred with BT, which decreased to ∼30%, presumably because of a significant increase of SHBG. In our cohort, this decline was ∼1% per year, similar to what was found in other studies [Citation14,Citation15].
The main objective of this research was to determine whether, in a public hospital, we could replace the total and free testosterone calculated by one of two questionnaires for screening and thus relieve the public health system from possible financial burden. Due to different structures and issues involved in each questionnaire, the results obtained were quite different.
The ADAM questionnaire is more efficient in assessing patients with testosterone deficiency. Moreover, worldwide, it is commonly used in the clinical practice. It was created from items aiming to diagnose the more prevalent symptoms in men presenting low testosterone levels, as defined by a Saint Louis University group in Ontario, Canada.
The testosterone levels were shown to be compatible with hypogonadism, with the calculated FT ≤65 pg/ml, indicating a sensitivity of 73.6%, whereas the specificity was 31.9%. These values are similar to results previously reported (sensitivity from 80.14% to 83.3% and specificity from 19.7% to 34.8%). Although the sensitivity is similar to that reported by Canadian physicians involved in the initial validation of ADAM questionnaire (88%), the specificity obtained in our study is significantly lower than the one described by Morley et al. (60%). We believe that this might reflect the difference between the two populations. In our study, we included male patients aged 40 years and older of a voluntary program for prostate cancer screening. In theory, men suffering from different urologic disorders would spontaneously seek medical evaluation. Thus, many individuals could have been included in the study in which their positive answers to one or more items of ADAM questionnaire might have been associated to co-morbidities that would have simulated the clinical symptoms of hypogonadism. Previous studies, carried out in a population presenting similar characteristics and low levels of specificity similar to those assessed in this work, revealed a high prevalence of depression in men with positive ADAM test and normal levels of calculated FT. Using the Carroll Depression Scale, patients classified as depressed showed a positive correlation with ADAM survey (r = 0.66, p < 0.05). Men with positive results in the ADAM questionnaire and normal levels of FT tend to exhibit low self-esteem, social performance (low score obtained with the quality of life questionnaire carried out by the World Health Organization) and education level, when compared with patients presenting normal levels of FT and negative ADAM scores. Although this aspect was not directly assessed, this might contribute to the results obtained in this study because the patients evaluated presented mainly low socio-economic and educational levels [Citation16–19].
In general, the results obtained with ADAM questionnaire in our cohort of male volunteers were unsatisfactory, mainly due to the low specificity achieved. Moreover, the questionnaire revealed an efficiency of 52.75%, meaning that a correct hypogonadic and non-hypogonadal classification was accomplished in only half of the patients. In keeping with this notion, the use of these tools to replace testosterone levels assessment in Brazil is not recommended, in agreement with results previously observed by other authors.
The MMAS was developed from an extensive epidemiological research conducted by Feldman et al. in the 1980s and 1990s, when they assessed 1709 males aged 40 years or more. This survey was performed in households and not in a hospital environment. The patients underwent hormone therapy and completed MMAS, which aimed to identify the main eight risk factors previously mentioned for testosterone deficiency. This study does not detect symptoms of hypogonadism [Citation20–22].
The results obtained with the MMAS questionnaire were lower than those obtained using the ADAM questionnaire. The sensitivity, using TT as a reference parameter, was 66.4% whereas the specificity was 46.1%. Using BT, the sensitivity achieved was 60.35% and the specificity was 42.9%, results similar to those obtained by Morley et al. (sensitivity of 60% and specificity of 59%). For calculated FT, the values observed were a bit lower: sensitivity of 59.9% and specificity of 42.9% ( and ) [Citation23].
The MMAS questionnaire also showed a poor performance in our male volunteers, as observed in the ADAM questionnaire. MMAS efficiency was only 51.4%. This, most certainly, limits its use as diagnostic tool for clinical practice in Brazil.
Further, the difference in populations could explain the variability of our results. In the original study, the patients were selected randomly and treated at home. It was assumed that they were healthy patients, contrary to our cohort formed by men seeking hospital care and suffering from basic co-morbidities that generated some confusion. Moreover, as in the original study, the results obtained were lower than those observed using the ADAM questionnaire. We believe this might be because of the higher number of questions in the MMAS that do not directly assess the symptoms of hypogonadism but rather the risks factors for ADAM.
The data found in our study confirms the extreme difficulty in developing a survey based on the signs and symptoms that might replace biochemical assessments. In a complex syndrome, such as the ADAM, hormone levels and physical and/or psychological complaints might reflect different and complementary aspects of the pathology. This perception puts in doubt, the validity of a single measurement of testosterone and the importance of the hormonal profile of the patient to diagnose hypogonadism. Eventually, the assessment of clinical symptoms or life quality surveys is the best way to understand these patients. For instance, changes in aging-related lifestyle might play an important role in our achievements as well as reduced physical activity and consequences on muscular strength, bone mineral density and lack of energy. Lastly, the decrease in testosterone levels varies among individuals. Each patient presents a different response that is difficult to evaluate, as the threshold for the appearance of symptoms of hypogonadism is still not clearly set.
Nevertheless, screening tests are often characterized by a high sensitivity and quite low specificity. Thus, screening tests might be considered as useful tools to identify patients that should not undergo further examinations; therefore, surveys that assess hypogonadism might be used in clinical practice.
Conclusions
Our survey showed that ADAM is a disease particularly prevalent in the society. The results obtained suggest that the ADAM and MMAS questionnaires show reasonable sensitivity in the detection of disease in patients aged 40 years and above, with low levels of calculated FT. However, because of their low specificity, these tools cannot replace laboratory assessments for hormone levels, despite their usefulness.
Declaration of interest
The authors report no declarations of interest.
References
- Vermeulen A. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72
- Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl 2009;30:1–9
- Shelton JB, Rajfer J. Androgen deficiency in aging and metabolically challenged men. Urol Clin NA 2012;39:63–75
- Andersson A-M, Jensen TK, Juul A, et al. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J Clin Endocrinol Metab 2007;92:4696–705
- Charlton R. Ageing male syndrome, andropause, androgen decline or mid-life crisis? J Men’s Health Gend 2004;1:55–9
- Morales A, Collier CP, Clark AF. A critical appraisal of accuracy and cost of laboratory methodologies for the diagnosis of hypogonadism: the role of free testosterone assays. Can J Urol 2012;19:6314–18
- Morales A, Spevack M, Emerson L, et al. Adding to the controversy: pitfalls in the diagnosis of testosterone deficiency syndromes with questionnaires and biochemistry. Aging Male 2007;10:57–65
- Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol 2008;159:507–14
- Vermeulen A. Hormonal cut-offs of partial androgen deficiency: a survey of androgen assays. J Endocrinol Invest 2005;28:28–31
- Anawalt BD, Hotaling JM, Walsh TJ, et al. Performance of total testosterone measurement to predict free testosterone for the biochemical evaluation of male hypogonadism. J Urol 2012;187:1369–73
- Winters SJ, Kelley DE, Goodpaster B. The analog free testosterone assay: are the results in men clinically useful? Clin Chem 1998;44:2178–82
- Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2001;86:724–31
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536–59
- Mohr BA, Guay AT, O’Donnell AB, et al. Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts Male Ageing Study. Clin Endocrinol 2005;62:64–73
- Morley JE, Kaiser FE, Perry HM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metab Clin Exp 1997;46:410–13
- Delhez M, Hansenne M, Legros J-J. Andropause and psychopathology: minor symptoms rather than pathological ones. Psychoneuroendocrinology 2003;28:863–74
- Blümel JE, Chedraui P, Gili SA, et al. Is the Androgen Deficiency of Aging Men (ADAM) questionnaire useful for the screening of partial androgenic deficiency of aging men? Maturitas 2009;63:365–8
- Tancredi A, Reginster J-Y, Schleich F, et al. Interest of the androgen deficiency in aging males (ADAM) questionnaire for the identification of hypogonadism in elderly community-dwelling male volunteers. Eur J Endocrinol 2004;151:355–60
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000;49:1239–42
- Smith KW, Feldman HA and McKinlay JB. Construction and field validation of a self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol 2000;53:703–11
- Feldman HA. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the massachusetts male aging study. J Clin Endocrinol Metab 2002;87:589–98
- Gray A, Feldman HA, McKinlay JB, et al. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 1991;73:1016–25
- Morley JE, Perry HM III, Kevorkian RT, et al. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas 2006;53:424–42