Abstract
Objective: To evaluate the relationship between testosterone levels and the metabolic syndrome (MS) in men older than 45 years.
Methods: Six hundred and sixty men (45–70 years) selected from 2906 participants of a population screening for prostate cancer were included in this study. Testosterone and the components of MS were assessed in all men. MS was diagnosed according to NCEP-ATP III criteria. Triglycerides (TG)/HDL-cholesterol (chol) index was calculated.
Results: The presence of MS was inversely associated with testosterone (χ2, p < 0.001), independently of age (OR 0.802, CI 95%: 0.724–0.887, p < 0.0001). Hypertension was the most frequent abnormality observed followed by elevated TG and waist circumference (WC). Testosterone correlated positively with HDL-chol (r: 0.14, p < 0.0001) and negatively with body mass index (BMI)(r: −0.29, p < 0.0001), WC (r: −0.26, p < 0.0001), TG (r: −0.20, p < 0.0001), TG/HDL-chol (r: −0.20, p < 0.0001), glucose (r: −0.11, p = 0.005) and MS score (r: −0.23, p < 0.0001).
Conclusions: Our results show that in men older than 45 years, as long as testosterone levels decline, the prevalence of MS increases, independently of age. The correlations found between testosterone and four of the five components of MS, as well as with BMI and TG/HDL-chol ratio, a surrogate marker of insulin resistance, suggest considering male hypogonadism as a determinant of developmental abnormalities typical of MS.
Introduction
The metabolic syndrome (MS) includes a number of pathological conditions that are associated with an increased risk of the development of cardiovascular diseases and type 2 diabetes. As well, aging and hypogonadism are associated with MS in men [Citation1]. In postmenopausal women in whom estrogen levels are very low, prevalence of MS increases along with aging [Citation2]. In aging men endogenous total testosterone levels decline with age, but it is not well established if the decline in androgens or the aging process itself is responsible of the increased risk of MS. Longitudinal studies show that low testosterone is related with a higher risk of developing MS, although the reverse condition is also possible. The factors responsible for this relationship are not completely understood [Citation3].
Some authors have demonstrated that patients with higher testosterone levels have fewer than three components of MS in comparison with those having lower testosterone values [Citation4,Citation5]. Rodriguez et al. [Citation6] in the Baltimore Longitudinal Study of Aging found that the prevalence of MS increased with age and that this condition was associated with lower androgens levels. They also found that lower testosterone along with lower SHBG levels predicted a higher incidence of MS.
It has also been described that the components of the MS are related to sex hormone levels and hypogonadism [Citation7]. Ding et al. [Citation8] in a meta-analysis showed a high prevalence of hypogonadism in diabetic men. Other studies also showed that low androgen levels may be a risk factor for diabetes [Citation9]. Among the components of MS, it has been found that central obesity is associated with reduced testosterone levels [Citation10]. These authors suggested that waist circumference (WC) is better predicting endogenous testosterone concentrations than body mass index (BMI) and waist/hip ratio and also showed the highest correlation with the components of MS.
Hypertension has been associated with hypogonadism as well [Citation7]. Svartberg et al. [Citation11] found that individuals with hypertension had lower testosterone than those without hypertension independent of age.
Lipid alterations are also part of MS. It has been suggested that testosterone levels are associated negatively with total cholesterol, low-density lipoprotein cholesterol and triglycerides, and positively with high-density lipoprotein cholesterol [Citation12]. Testosterone replacement also showed an improvement in lipid profile [Citation13].
Taking into account the previous considerations, the aim of this study was to evaluate the relationship between testosterone levels and the MS in adult men older than 45 years.
Methods
Subjects
A total number of 2906 men (ages 45–70) completed a prostatic evaluation at the Urology Division, Hospital de Clínicas ‘‘José de San Martín’’, University of Buenos Aires, in May 2009, in the context of a population screening for the early detection of prostate cancer. For the purpose of the present study, we selected a subsample of 660 men in which testosterone levels were determined and the components of the MS could be assessed. Patients with hormonal therapy or any other drug-modifying lipid metabolism were excluded from the study.
All men gave their informed consent and the original screening study protocol was approved by the Ethics Committee of the hospital.
Biomedical measures
In order to calculate the BMI, weight and height were obtained for each patient. WC was measured at the level midway between the lateral lower rib margin and the superior anterior iliac crest, in a standing position. The biomedical measures were performed by nurses, always under the supervision of the same investigator. Blood pressure was registered in sitting position.
Analytical methods
Blood samples were obtained by venipuncture after 12 h fasting. Serum samples were separated by centrifugation at 1500 × g for 5 min; glucose was measured within the same day. For lipid and lipoprotein determinations, serum was kept at 4 °C until processing, within 48 h. A serum aliquot was stored at −70 °C for hormonal determinations.
Total testosterone was determined by a chemoluminiscent method (Immulite Autoanalyzer, Siemens, Los Angeles, CA). Total variation coefficients for testosterone were 16.4% (2.4 nmol/L) and 7.7% (27.4 nmol/L). Triglycerides (TG), total-cholesterol (chol), HDL-chol and glucose were measured in a Hitachi 917 autoanalyzer by enzymatic colorimetric methods (Roche Diagnostics GmbH, Mannheim, Germany). Intra and interassay variation coefficients were 1.3% and 2.4% (TG), 1.1% and 2.2% (chol), 1.5% and 2.6% (HDL-chol), and 1.1% and 2.2% (glucose), respectively. We also calculated the ratio of TG/HDL-chol, which is considered a surrogate marker of insulin resistance [Citation14]. The MS was diagnosed according to the National Cholesterol Education Program (NCEP), Adult Treatment Panel-III (ATP III) [Citation15]. Patients were classified as having MS if they met three or more of the following criteria: WC >102 cm, TG ≥1.7 mmol/L, HDL-cholesterol <1.03 mmol/L, systolic and/or diastolic pressure ≥130/85 mmHg, and impaired fasting glucose ≥6.1 mmol/L. The MS score considered the number of components that each patient presented, according to the NCEP ATP III.
Statistical analysis
In order to analyze data distribution, the Kolmogorov–Smirnoff test was used. The relation between testosterone levels and the presence of MS was analyzed by χ2-test. Correlations between variables were calculated using the Spearman test. In order to test whether testosterone levels contributed to explain the prevalence of MS in this population, beyond the effects of age, multiple regression analysis was performed. Statistical analysis was performed using SPSS 19 software (SPSS, Chicago, IL).
Results
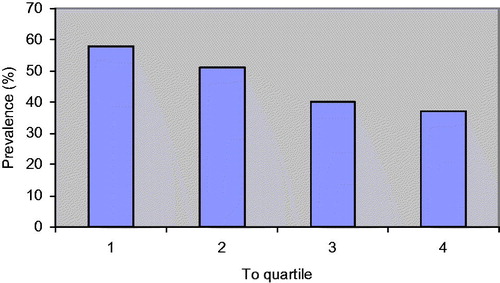
Patients were divided into four groups according to testosterone quartiles (low T, low-normal T, mid-normal T and high-normal T), which were created by dividing testosterone values of the whole population into four groups of similar size. The presence of MS showed an inverse association with testosterone concentrations (χ2, p < 0.001) ( and ).
Table 1. Association between testosterone levels and the presence of MS.
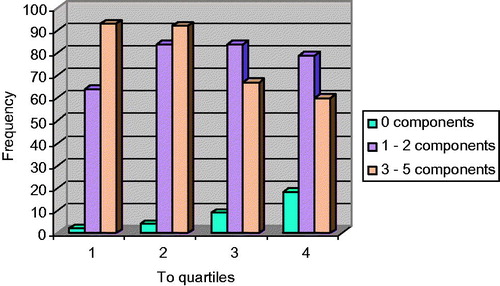
shows the distribution of subjects with different number of components of the MS. According to the increase in testosterone levels, the number of patients with three or more MS components decreased (χ2, p < 0.0001). The prevalence of MS is higher in patients with low testosterone levels.
Figure 2. Frequency distribution according to the number of components of MS in relation to testosterone (To) quartiles in the population studied (χ2, p < 0.0001).
We analyzed the prevalence of each of the components of the MS in the population studied. Hypertension was the most frequent abnormality observed followed by triglycerides ≥1.7 mmol/L and WC >102 cm ().
Table 2. Distribution of components of the MS in the population studied.
A positive and significant correlation was found between testosterone and HDL-chol. The best negative correlations were noted between testosterone and BMI and WC, while the weakest correlation was seen between testosterone and glucose (). We did not find a significant correlation between testosterone and hypertension.
Table 3. Correlation between testosterone levels and different parameters.
Testosterone concentrations also showed a significant and negative correlation with MS score (r = −0.23, p < 0.0001). We then performed a multiple regression analysis which showed that testosterone predicts MS independently of age (OR 0.802, CI 95%:0.724–0.887, p < 0.0001).
Discussion
In our study, the prevalence of MS increased as well as testosterone levels become lower. Considering that testosterone levels decreases with age in normal men, this situation must be taken into account when physicians consider risk of cardiovascular disease and type 2 diabetes in this population. In this regard, we were able to show that as testosterone levels decrease, the presence of MS increases, independently of age.
The MS is considered a cluster of related risk factors that lead to a higher incidence of cardiovascular diseases [Citation16]. In our study the higher incidence of the MS in subjects with lower testosterone levels was associated with abdominal obesity, a condition that was present in 40% of the men studied. Many studies reported an inverse association between total testosterone levels and obesity or waist circumference in men [Citation10,Citation17–20]. It has been postulated that a possible mechanism to explain this issue is the increased aromatase activity found in visceral and peripheral fat that diminishes testosterone levels by conversion in estradiol, which in turn inhibits luteinizing hormone (LH) levels at the pituitary with the consequent decrease of testosterone production by the Leydig cells. Another explanation that may account for the decrease in testosterone levels in obese men involves elevated serum leptin levels in individuals with large fat reserves that may interfere with LH stimulated androgen biosynthesis [Citation21]. Leydig cells express leptin receptors and the binding of leptin attenuates these cells response to LH [Citation22]. High leptin levels may also interfere with the KISS 1/kisspeptin system, a hormonal central regulatory mechanism that controls Gn-RH secretion and thus testicular steroidogenesis [Citation22]. Another link between hypogonadism and obesity may consider insulin resistance (IR), which has also been shown to reduce circulating androgen levels [Citation23]. In addition, cytokine-mediated inhibition of testicular testosterone production in obesity may also play a role [Citation18]. Kaplan et al. examined testosterone levels in 864 subjects with and without MS and found that obese men with MS had significantly lower testosterone levels than non-obese men with MS [Citation5]. Although we have not performed a multivariate regression analysis, our results are in accordance with those of Kaplan et al., and also show an inverse relationship between testosterone levels and BMI in the men studied. This is in accordance with other authors [Citation17], although WC is considered a better predictor of testosterone levels than BMI [Citation10].
Although the ATP III criteria do not consider evaluation of IR to establish the presence of MS, this alteration is presumed to be the main defect leading to it [Citation16]. An inverse relationship between testosterone levels and IR has been found [Citation21,Citation24–26]. Osuna et al. [Citation21] observed negative correlation between WC, BMI, insulin and HOMA-IR with testosterone levels. In the Kuopio Ischaemic Heart Disease Risk Factor Study, Laaksonen et al. [Citation17] evaluated 1896 non-diabetic middle-aged men and found that individuals with MS had elevated insulin levels and decreased total testosterone when compared with controls. In addition, Muller et al. [Citation27] concluded that elevated testosterone and SHBG led to increased insulin sensitivity and reduced risk of MS. Moreover, Pagotto et al. [Citation28] described that hypogonadism was a stronger risk factor for the development of elevated insulin and glucose levels compared with overweight/obesity. Low testosterone levels found in the cases of IR may indicate a defect at one or more functional levels of the hypothalamic-pituitary-gonadal axis. In the IR state, Leydig cell function, particularly steroidogenesis, may be impaired by changes in the production of hormones and cytokines locally in the target tissue and the adipose tissue [Citation23,Citation25,Citation26]. Difficulties in the assessment of IR by the gold standard method (i.e. the hyperinsulinemic euglucemic clamp) are well known, so we consider in this study the use of a secondary marker of IR as the ratio of TG/HDL-chol [Citation14] to test if there was an association between testosterone levels and the MS. We found that testosterone levels correlated negatively with TG/HDL-chol, which is suggestive of an inverse relation between testosterone and IR.
Hypertension has also been associated with hypogonadism [Citation7]. Svartberg et al. [Citation10] found that individuals with hypertension had lower testosterone values than those who were not hypertensive. Moreover, other authors found that androgen deprivation therapy in men with prostate cancer could also induce hypertension and arterial stiffness [Citation29]. However, in this study, we could not demonstrate any association between testosterone levels and elevated blood pressure. Our results are in accordance with those of Chubb et al. [Citation30] in a cross-sectional study of 2502 older men, where total testosterone was shown to be associated with all MS components except hypertension. These authors found that 88.5% of the men studied had elevated blood pressure, this percentage being very similar to our results (82.6%).
Regarding the relation between lipids and androgens, low levels of testosterone have been associated with an atherogenic lipoprotein profile, characterized by high LDL-chol and triglyceride levels [Citation31]. Several studies have suggested that reduced testosterone levels are associated with increased total cholesterol and LDL-chol [Citation32,Citation33]. A positive association between HDL-chol levels and testosterone was described in several studies [Citation34,Citation35]. This is in accordance with our results, however, other authors described no change [Citation13,Citation36] or even a decrease in HDL-chol after testosterone treatment [Citation37]. In a study from our laboratory, we have previously reported that hypogonadal men presented a decrease in HDL-chol after six months of i.m. treatment with testosterone, although HDL-chol retrieved baseline levels after 18 months of treatment [Citation38].
In studies in patients with prostate cancer, treated with androgen deprivation therapy, there was an increment in all the lipids parameters studied, total cholesterol, LDL-chol, HDL-chol and triglycerides [Citation29,Citation39]. On the contrary, another study reported that men under androgen supplementation therapy had decreased total cholesterol, as well as LDL-chol and triglycerides, but increased HDL-chol, and the effect was greater with the long-acting testosterone formulation in contrast to the testosterone gel [Citation35]. Recent data indicate that in addition to quantity, HDL quality is also very important in the protection from atherosclerosis and cardiovascular disease [Citation40]. It is suggested that not only HDL-chol levels must be high, but this must be accompanied with an improvement of the atheroprotective potential of this lipoprotein, including antioxidant behavior and increased efficiency of reverse cholesterol transport [Citation41].
In summary, androgen deficiency contributes to increased triglycerides, total cholesterol, LDL-chol and reduced HDL-chol while androgen treatment results in a favorable lipid profile, suggesting that androgens may provide a protective effect against the development and/or progression of atherosclerosis [Citation41].
Evidence linking androgen deficiency to multiple risk factors including obesity, diabetes, hypertension and altered lipid profiles is reinforced by the effects produced by androgen therapy. It has been demonstrated that testosterone treatment significantly improves lipid profiles in men, as mentioned above, reduces body fat percentage, increases lean muscle mass percentage and strength, as well as bone mineral density, lowers blood pressure and decreases fasting glucose levels, slowing or halting the progression of MS, type 2 diabetes and cardiovascular disease [Citation1,Citation42].
One limitation of this study is the fact that this was a retrospective analysis of testosterone and MS data from a sub-cohort (n = 660) of men from a single center participating in a prostate cancer screening study.
In conclusion, our results show that in this group of men older than 45 years, as long as testosterone levels decline, the prevalence of MS increases. The correlations found between testosterone and 4 of the 5 components of MS, as well as with BMI and TG/HDL-chol ratio, a surrogate marker of IR, suggest considering male hypogonadism as a determinant of developmental abnormalities typical of MS.
Declaration of interest
The authors report no declarations of interest.
This work was supported by grants from University of Buenos Aires (B401 and 20020110100041).
References
- Traish AM, Guay A, Feeley R, Saad F. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl 2009;30:10–22
- Mesch VR, Boero LE, Siseles NO, et al. Metabolic syndrome throughout the menopausal transition: influence of age and menopausal status. Climacteric 2006;9:40–8
- Corona G, Rastrelli G, Morelli A, et al. Hypogonadism and metabolic syndrome. J Endocrinol Invest 2011;34:557–67
- Blouin K, Despres JP, Couillard C, et al. Contribution of age and declining androgen levels to features of the metabolic syndrome in men. Metabolism 2005;54:1034–40
- Kaplan SA, Meehan AG, Shah A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively high incidence of erectile dysfunction observed in these men? J Urol 2006;176:1524–8
- Rodriguez A, Muller DC, Metter EJ, et al. Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab 2007;92:3568–72
- Guay A. The emerging link between hypogonadism and metabolic syndrome. J Androl 2009;30:370–6
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006;295:1288–99
- Selvin E, Feinleib M, Zhang L, et al. Androgens and diabetes in men. Diabetes Care 2007;30:234–8
- Svartberg J, von Muhlen D, Sundsfjord J, Jorde R. Waist circumference and testosterone levels in community dwelling men. The Tromso Study. Eur J Epidemiol 2004;19:657–63
- Svartberg J, von Muhlen D, Schirmer H, et al. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromso Study. Eur J Endocrinol 2004;150:65–71
- Maggio M, Basaria S. Welcoming low testosterone as a cardiovascular risk factor. Int J Impot Res 2009;21:261–4
- Malkin CJ, Pugh PJ, Jones RD, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 2004;89:3313–18
- McLaughlin T, Abbasi F, Cheal K, et al. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med 2003;139:802–9
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive summary of the third report of the National Cholesterol Education Program (NCEP). JAMA 2001;285:2486–97
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;16:1415–28
- Laaksonen DE, Niskanen L, Punnonen K, et al. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol 2003;149:601–8
- Kalyani RR, Dobs AS. Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr Opin Endocrinol Diabetes Obes 2007;14:226–34
- Lunenfeld B, Mskhalaya G, Kalinchenko S, Tishova Y. Recommendations on the diagnosis, treatment and monitoring of late-onset hypogonadism in men – a suggested update. Aging Male 2013;16:143–50
- Lunenfeld B, Arver S, Moncada I, et al. How to help the aging male? Current approaches to hypogonadism in primary care. Aging Male 2012;15:187–97
- Osuna JA, Gomez-Perez R, Arata-Bellabarba G, Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch Androl 2006;52:355–61
- Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol 2009;5:673–81
- -Traish AM, Saad F, Guay A. The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance. J Androl 2009;30:23–32
- Tsai EC, Matsumoto AM, Fujimoto WY, Boyho E. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care 2004;27:861–8
- Pitteloud N, Hardin M, Dwyer AA, et al. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab 2005;90:2636–41
- Pitteloud N, Mootha VK, Dwyer AA, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 2005;28:1636–42
- Muller M, Grobbee DE, den Tonkelaar I, et al. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab 2005;90:2618–23
- Pagotto U, Gambineri A, Pelusi C, et al. Testosterone replacement therapy restores normal ghrelin in hypogonadal men. J Clin Endocrinol Metab 2003;88:4139–43
- Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab 2006;91:1305–8
- Chubb SA, Hyde Z, Almeida OP, et al. Lower sex hormone-binding globulin is more strongly associated with metabolic syndrome than lower total testosterone in older men: the Health in Men Study. Eur J Endocrinol 2008;158:785–92
- Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev 2003;24:183–217
- Simon D, Charles MA, Nahoul K, et al. Association between plasma testosterone and cardiovascular risk factors in healthy adult men: the Telecom study. J Clin Endocrinol Metab 1997;82:682–5
- Barud W, Palusinski R, Beltkowski J, Wojcicka G. Inverse relationship between total testosterone and anti-oxidized low-density lipoprotein antibody levels in ageing males. Atherosclerosis 2002;164:282–8
- Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab 2007;92:3844–53
- Saad F, Gooren LJ, Haider A, Yassin A. A dose–response study of testosterone on sexual dysfunction and features of the metabolic syndrome using testosterone gel and parenteral testosterone undecanoate. J Androl 2008;29:102–5
- Allan CA, Strauss BJG, Burger HG, et al. Testosterone therapy prevents gain in adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab 2008;93:139–46
- Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older age: a randomized controlled trial. JAMA 2008;299:39–52
- Berg G, Schreier L, Geloso G, et al. Impact on lipoprotein profile after long term testosterone replacement in hypogonadal men. Horm Metab Res 2002;34:87–92
- Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 2002;87:599–603
- Tsompanidi EM, Brinkmeier MS, Fotiadou EH, et al. HDL biogenesis and functions: role of HDL quality and quantity in atherosclerosis. Atherosclerosis 2010;208:3–9
- Traish A, Abdou R, Kypreos KE. Androgen deficiency and atherosclerosis: the lipid link. Vascul Pharmacol 2009;51:303–13
- Bhattacharya RK, Khera M, Blick G, et al. Testosterone replacement therapy among elderly males: the Testim Registry in the US (TRiUS). Clin Interv Aging 2012;7:321–30