Abstract
Aging is associated with erectile dysfunction (ED), in which nitric oxide synthase (NOS) activity and NO bioavailability are reduced due to deficiencies of NOS cofactor (tetrahydrobiopterin, BH4) and substrate (l-arginine). We determined whether the prolonged treatment with sodium nitrite (NaNO2) as a storage form of NO ameliorates ED in aged rats. Male Sprague–Dawley rats were divided: younger, aged and NaNO2-treated (20 mg/kg per day) aged groups. The erectile (intracavernosal pressure [ICP]/mean arterial pressure [MAP]) and corpus cavernous (CC) responses were evaluated after 12 weeks. The ICP/MAP in aged rats was lower than in young controls, which was not improved by the NaNO2 treatment. Immunohistochemical (IHC) staining for endothelial NOS and collagen deposition was performed. We assayed NO indirectly by measuring the level of its stable end products, nitrite/nitrate, using the Griess reagent. The relaxations to ACh and EFS in the aged group were considerably less than in the younger group, which were normalized by acute incubations of l-arginine or BH4 of aged CC. In conclusion, NaNO2 treatment did not restore erectile response while nitrate levels were enhanced in aged penis. The cofactor or substrate administrations, but not chronic exogenous modulation of NO system may be beneficial in aged men with ED.
Introduction
Age-related diseases are the greatest health challenge in the industrialized countries [Citation1]. Erectile dysfunction (ED) increased greatly in older males [Citation2,Citation3]. The Massachusetts Male Aging Study, 34.5% of men aged 40–70 years old have moderate-to-complete ED, and 15% of men aged 70 years old have complete ED [Citation4].
When ED is assessed in the older patient, it is important to recognize and treat underlying systemic disorders. Aging is commonly associated with increasing degrees of atherosclerotic vascular alteration in the arterial bed of the penis [Citation5]. The vascular supply to the penis is compromised in the aging male [Citation6]. The medial thickening and reduced capacity for penile blood flow have been shown by histological experiments [Citation6]. Hence, diminished nerve and endothelium-mediated corpus cavernosum (CC) relaxation [Citation7], decreased androgen levels [Citation8], pathological remodeling of the pudendal artery [Citation6] reduction in smooth muscle content [Citation9–12], impaired growth factor and cytokine signaling [Citation3,Citation13] and up-regulation of the RhoA/Rho-kinase contractile pathway [Citation14] have been demonstrated in the penis in age-related ED. On the other hand, decreased endothelial signaling is attributed to impaired endothelial nitric oxide synthase (eNOS) expression [Citation15–18], decreased eNOS phosphorylation [Citation18], decreased content of NOS substrate l-arginine [Citation19], excessive cyclic guanosine monophosphate (cGMP) degradation by upregulated phosphodiesterase-5 (PDE5) [Citation20], reduced activation of protein kinase G by cGMP [Citation21] and increased oxidative stress [Citation22]. Nitrite (NO2) may represent a circulating and tissue storage form of NO. Tomada et al.'s study [Citation23] demonstrated that aging caused by a significant reduction of smooth muscle-to-connective tissue ratio in CC. The main vascular growth factor, vascular endothelial growth factor (VEGF) plays an important role in the endothelial cell survival and proliferation; control vascular biology and stimulates NO production [Citation24]. However, VEGF expression markedly decreased in aged human CC tissue [Citation25], while the expression of angiopoietins increased in the aged CC [Citation26]. It appears that the vascular endothelium and smooth muscle components of cavernosal tissue during aging can be modified by many factors.
Sodium nitrite (NaNO2) acts as a storage form of NO and has beneficial pharmacological actions and is converted to vasoactive NO in the CC and systemic vascular bed [Citation27]. The intracavernosal injection of NaNO2 produced dose-related increases in intracavernosal pressure (ICP) and decreased in systemic arterial pressure [Citation27]. Recently, Lasker and co-workers demonstrated that NaNO2 served as a NO donor and increased erectile activity in the rat [Citation27]. In this study, we aimed to evaluate whether treatment with NaNO2 ameliorates ED in aged rats.
Methods
Animals
Male Wistar rats were divided into three groups: (1) Young (3-month-old, n = 6), (2) aged (24-month-old, n = 6) and (3) NaNO2-treated aged rats (n = 6). Group 3 received NaNO2 (50 mg/L) in their drinking water for 12 weeks. Body weights were measured every week during the study and before experiments which were performed after 12 weeks. All animals were housed in separate cages and provided with food and water ad libitum in a temperature-controlled room (22 ± 1 °C), artificially lit from 7:00 AM to 7:00 PM daily. This study was approved by the Ethics Committee of Animal Care of Ankara University.
Erectile function measurement in in vivo studies
To measure ICP, the rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). The trachea was cannulated (polyethylene, [PE]-240 tubing) to maintain a patent airway and the carotid artery was cannulated (PE-50 tubing) to measure main arterial pressure (MAP) using a transducer (Statham, Oxnard, CA) attached to a data acquisition system (Biopac MP 100 System, Santa Barbara, CA). A 25-G needle filled with 250 U/mL of heparin and connected to the PE tubing was inserted into the right crura of the penis and connected to the pressure transducer to measure continuously ICP. The right major pelvic ganglion and CN were identified. A stainless-steel bipolar hook electrode for stimulation was placed around the CN postero-lateral to the prostate on one side. The MAP and ICP were continuously measured, and the CN was stimulated (2.5, 5 and 7.5 V, 15 Hz, 30 s train duration) with a square-pulse stimulator (Grass Instruments, Quincy, MA).
Cavernous tension measurement in in vitro studies
Isolated CC strips were maintained in Krebs-bicarbonate solution (containing [in mM] NaCl: 118.1; KCl: 4.7; KH2PO4: 1.0; MgSO4: 1.0; NaHCO3: 25.0; CaCl: 22.5; and glucose: 11.1). The organ bath temperature was maintained at 37 °C via a circulating water bath. Oxygen saturation and a pH of 7.4 were maintained by continuous air with a mixture of 95% O2, 5% CO2. After placement in the organ chamber, the preparations were allowed to equilibrate for a minimum of 60 min and the bath solution was replaced every 15 min. Each tissue strip was submaximally contracted by adding phenylephrine (Phe, 10−5 M) into the organ chamber. Electrical field stimulation (EFS) of autonomic nerves (duration: 15 s; amplitude: 50–90 V; frequency range: 1–20 Hz; pulse width: 0.5 ms) was accomplished by means of platinum electrodes, positioned on the either side of the tissue (Grass Instruments, Quincy, MA) similar to our earlier study [Citation28]. EFS-induced nitrergic relaxation response was evoked after pre-contraction of Phe (10−5 M) in CC strips. After stabilization of the Phe (10−5 M)-induced pre-contraction, relaxations to ACh (10−8–10−3 M) and SNP (10−8–10−3M) were induced in the CC strips. The relaxations to ACh and EFS were repeated by acute incubations of l-arginine (10−4 M) or tetrahydrobiopterin (BH4, 10−5 M). For EFS response curves, the CC strips were pre-incubated for 30 min with guanethidine (5 mM) to eliminate noradrenergic influences, and atropine (1 mM) to prevent cholinergic responses. After Phe pre-contraction under these conditions, EFS produced only relaxation responses mediated by nitrergic (or named as non-adrenergic non-cholinergic with the old expression) fibers [Citation28,Citation29].
In addition, cumulative dose–response curves for Phe (10−8–10−3 M) contraction were examined. The direct sympathetic stimulation was induced neurogenically between 1 and 20 Hz in the CC strips.
Masson's trichrome staining
The proportions of collagen and muscle in the tissue samples were determined by staining tissue sections with Masson's trichrome, staining the extracellular matrix (collagen and connective tissue elements) blue and smooth muscle red as in previous studies [Citation23,Citation29]. As collagen represents the majority of the extracellular matrix, blue staining areas were referred to as stained collagen. Bouin's Solution (Sigma Chemical Co., #HT10-1, St. Louis, MO) pre-heated at 56 °C for 15 min or at room temperature overnight was used as a mordant in the tissue sections. The slides were then cooled with tap water (18–26 °C) in a Coplin jar and washed in running tap water to remove the yellow color. Slides were stained in working Weigert's Iron Hematoxylin Solution for 5 min and then washed in running tap water for 5 min. Thereafter, tissues were rinsed in deionized water and stained with Biebrich Scarlet-Acid Fucshin (Sigma Chemical #HT15-1) for 5 min, then in Working Phosphotungstic/Phosphomolybdic Acid Solution (Sigma Chemical #HT15-3) for 5 min, followed by Aniline Blue Solution (Sigma Chemical #HT15-4) for 5 min, and then 1% acetic acid for 2 min. Finally, the tissues were rinsed and dehydrated with alcohol, cleaned with xylene, and mounted. To avoid variations in staining, all of the reagents were prepared in one batch, and Masson's trichrome staining was performed on all CC tissues at the same time. Image analyses of the Masson's trichrome stains were performed using the Image-J software to identify the two distinct populations of collagen and smooth muscle fibers as published [Citation8]. The mean proportion of collagen and muscle fiber and standard errors of the means for the entire tissue section were calculated from multiple readings.
Semiquantitative immunohistochemistry for eNOS
Bivalved penises were fixed in 10% formalin and processed for paraffin embedding. Tissue cross sections (8–10 µm) were deparaffinized in xylene and hydrated with graded alcohol. Endogenous peroxidases were quenched with 3% hydrogen peroxide, and sections were washed with phosphate buffered saline (PBS). Non-specific binding (immunoglobulin G) was blocked using normal horse serum (1:50 dilution) in 0.1% bovine serum albumin in PBS. Slides were treated with 0.1% Triton X-100 for 20 min, washed in PBS for 5 min and then incubated with rabbit primary polyclonal antibody (anti-eNOS; BD Transduction Laboratories, San Diego, CA) at a dilution of 1:100 for 1 h at room temperature. Samples were then washed and incubated for an additional 30 min with biotinylated secondary antibody (DAKO, Carpinteria, CA). Following a further 30-min incubation with an avidin–biotin-conjugated horseradish peroxidase (DAKO), the DAB substrate was added for 5 min. The eNOS-positive cells appeared brown against Harris hematoxylin counterstain. Negative control slides, stained with only secondary antibody, were carried out for each tissue specimen (data not shown). Six to eight sections were employed for semiquantitative analysis of eNOS staining from each of the five penile tissues. Images were visualized under light microscopy, and image analysis was performed using Image-J to identify the staining intensity of the immunohistochemical reaction.
Nitrate and nitrite (NOx) analysis
Nitrate and nitrite concentrations were calculated with a Nitrate/Nitrite Colorimetric Assay kit (Cayman Chemical Company, USA) and measured with the Griess method. Penile tissue samples were homogenized in PBS, pH 7.4, and centrifuge at 10 000 × g for 20 min, and then the supernatant solution was ultracentrifuged at 100 000 × g for 30 min. After being passed through 30-kD ultrafilters (Millipore, Bedford, MA), 40 mL of the tissue lysates was diluted with 240 mL assay buffer and mixed with 10 mL cofactor and 10 mL nitrate reductase (colorimetric assay kit, Cayman Chemical Co). After the samples had been kept at room temperature for 3 h to convert nitrate to nitrite, total nitrite was measured at 540 nm absorbance by reaction with Griess reagent (sulfanilamide and naphthalene–ethylene diamine dihydrochloride) in a microplate reader to obtain nitrite plus nitrate concentrations. Amounts of nitrite in the samples were estimated by a standard curve obtained from enzymatic conversion of sodium nitrate (NaNO3) to nitrite.
Analysis of data and statistics
Values were expressed as mean ± SEM. Statistical differences were determined by analysis of variance followed by Bonferroni's complementary analysis using the Prism GraphPad 4 (San Diego, CA). A p value < 0.05 was considered to be significant. At the end of the experiments, each CC strip was weighed. All contractile responses were expressed as milligrams of tension developed per milligram of CC strips.
Drugs
All drugs were purchased from Sigma Chemical Co (St. Louis, MO).
Results
Body weights in groups
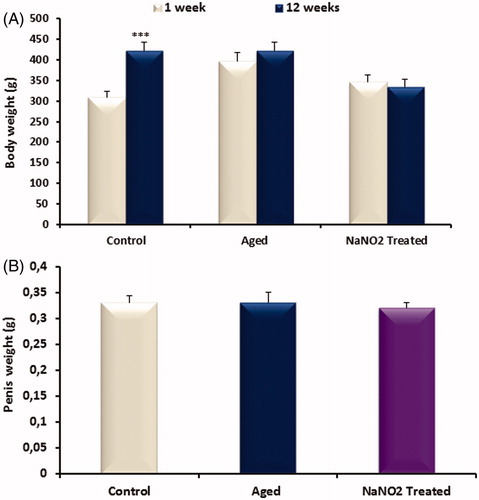
The final body weight of the control group at 12 weeks was significantly increased when compared to initial weight (p < 0.001; ). There was no difference in body weight of aging rats and NaNO2-treated rats after 12 weeks. Penis weight was not different among groups ().
ICP/MAP and total ICP values in groups
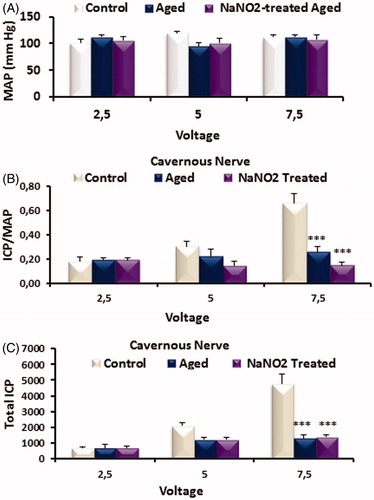
While MAP values were not altered in groups (), ICP/MAP () and total ICP () values by stimulation of CN at 7.5 V levels were decreased in aged rats as compared to young rats, which was not reversed by the treatment. There was no alteration at 2.5 and 5 V levels among groups (p > 0.05; ).
Figure 2. Bar graph showing MAP (A), ICP/MAP (B) and total ICP (C) responses for cavernous nerve (CN) in control, aged control and NaNO2-treated groups. Abbreviations: ICP, intracavernosal pressure; MAP, mean arterial pressure; CN, cavernous nerve. Data are mean ± SEM (n = 6). ***p < 0.001 versus control.
EFS-induced relaxation responses on CC strips
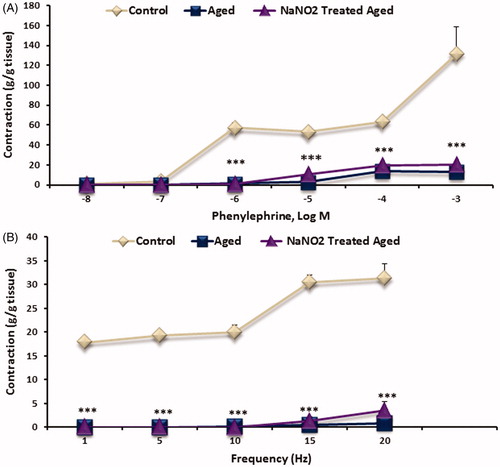
As shown in , the relaxation response induced by EFS was increased significantly in aged rats when compared to young rats (p < 0.001). In treating rats, median stimulations (such as 5, 10 and 15 Hz) were partially reversed as seen in . While the relaxation response to EFS after incubation with l-arginine was partially reversed at 5 Hz, it was observed complete improvement with BH4 incubation (p < 0.001, ).
Figure 3. Dose–response graphs showing in CC strips to EFS (1–20 Hz frequency, A); ACh (10−8–10−3 M, B) induced relaxation. Data are mean ± SEM (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001 versus control. Abbreviations: Phe, phenylephrine; ACh, acetylcholine; EFS, electrical field stimulation.
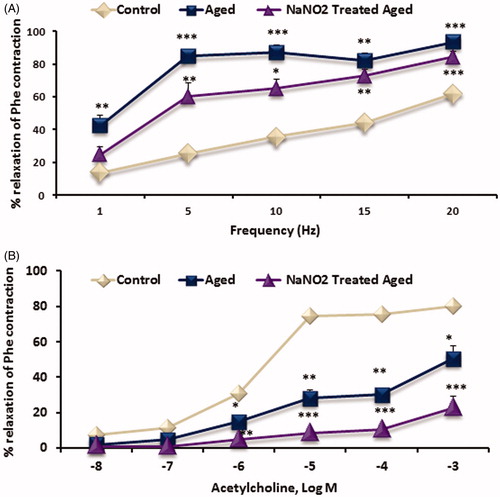
Table 1. The relaxation responses to EFS (1, 5 and 10 Hz) in the absence or presence of l-arginine or BH4 from young and aged rats.
ACh-induced relaxation responses on CC strips
ACh-induced endothelial-dependent relaxation response was significantly reduced in aging rats (p < 0.001; ). CC strips from treated rats did not show any improvement in the relaxant responses of ACh. After incubation of l-arginine or BH4, relaxation to ACh was partially corrected in aged and NaNO2-treated rats (p < 0.05, ).
Table 2. The relaxation responses to acetylcholine (ACh; 10−3) in the absence or presence of l-arginine or BH4 in corpus cavernosum from young, aged and NaNO2-treated aged rats.
SNP-induced relaxation responses on CC strips
SNP-induced relaxation responses were served normally in all groups (data not shown).
Phe-induced contractile responses on CC strips
Phe-induced contractile responses were significantly reduced in aged rats when compared to younger rats (p < 0.001; ). Treatment with NaNO2 did not reverse the decreased on the contractile response of Phe in aged group.
EFS-induced contractile responses on CC strips
In aged rats, EFS-induced noradrenergic contractile responses were significantly reduced (p < 0.001), which were not improved by the NaNO2 treatment ().
Smooth muscle-collagen ratios
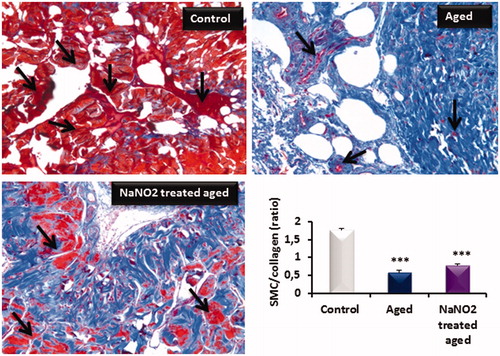
The Masson's trichrome staining procedure was used to determine the relative area of collagen to muscle fibers in the cavernosal tissue. The staining method revealed a ratio of smooth muscle cell (SMC)/collagen, which was markedly different between in aged rats when compared to young rats (). In aged tissue samples, the SMC/collagen ratio decreased, which was not returned by NaNO2 treatment as seen bar graphic of .
Figure 5. Masson's trichrome staining of cavernous tissue from young control, aged and NaNO2-treated aged rats. Smooth muscle and connective tissues are stained in red and blue, respectively. Smooth muscle cell (SMC)/collagen ratio determined by Masson trichrome staining on paraffin-embedded penile tissue sections was shown in the lower bar graphic. Please notice blue areas were markedly enhanced in aged rats when compared to control rats. However NaNO2 treatment slightly enhanced red areas in aged samples, but it was not significant when compared to control samples (***p<0.001).
eNOS immunostaining in groups
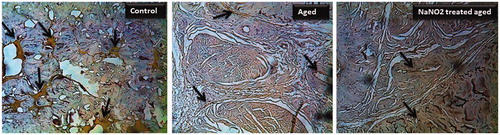
In order to elucidate the cellular expression of eNOS, CC tissue sections were immunohistochemically stained with anti-eNOS antibody, and results were presented in . There was a weak expression of eNOS protein in the CC of aging rats when compared to control rats (). Treatment with NaNO2 of aging rats did not alter the expressions of eNOS ().
Figure 6. Immunohistochemical localization of eNOS in rat penis (40 × magnification) from young control, aged and NaNO2-treated aged rats. Please notice the eNOS staining (dark brown) with decreased localization to the cavernous and vascular areas from aged groups. The negative control section processed without antibodies did not stain (data not shown).
NOx levels in corpus cavernosum
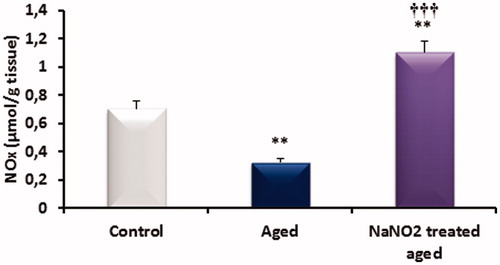
NOx levels in the CC of the three groups are shown in . The penile tissue levels of nitrate plus nitrite (NO metabolites) of aged rats were significantly lower than those control rat samples. However, NOx levels NaNO2-treated aged rats significantly increased when compared untreated aged and control rats as shown in .
Discussion
In this study, we showed that (1) in vivo erectile activity by stimulation of the CN in aged and NaNO2-treated rats was significantly diminished, while the relaxation responses to EFS were higher than young control rats in isolated CC strips from aged and treated rats; (2) ACh-induced relaxation response and eNOS immunostaining were significantly decreased, which was not improved by the treatment accompanied by increased levels of tissue NO metabolites in aged rat penile samples; (3) the contractile responses to α1-adrenergic agonist and neurogenic stimulation in CC from aged and treated rats were diminished; (4) acute incubation with l-arginine or BH4 partially returned diminished ACh responses and enhanced EFS-induced relaxation in aged isolated CC tissues.
Penile erection can be measured by CN stimulation in anesthetized rats. In this study, we observed that the erectile response of CN in aged rats was remarkably diminished, which was not restored by the treatment with NaNO2. The relaxation produced by SNP, a NO donor, was not changed by physiological aging. This detrimental effect of aging on CC function is not related to an increase in arterial blood pressure because, in our experimental conditions, MAP was not significantly affected as a consequence of aging. Previous studies in aging rats showed a reduction of erectile response [Citation14,Citation18,Citation30]. In addition, in this study, in vitro nitrergic relaxant response to EFS of isolated CC from aged and NaNO2-treated group was higher than from the young control group. We presented that despite of decreased in vivo responses, EFS-induced relaxant responses in isolated CC were enhanced. However, previous studies showed that EFS-induced relaxant responses were reduced in isolated CC from aged rats [Citation30,Citation31]. In this study, CC strips from aged rats were incubated with NO substrate (l-arginine) or co-factor (BH4) prior to relaxation to EFS. The nitrergic relaxation response was partially reversed by the NO substrate l-arginine at median frequencies. In the previous study by Angula et al., l-arginine has no ability to relax rabbit corpus cavernosum [Citation32]. Our earlier observations demonstrated that l-arginine induced slow and prolonged relaxation in human CC [Citation33]. We investigated the relaxant effects of repetitive administration of l-arginine, the substrate for NO, at hourly intervals and elucidated its mechanism of action in human corpus cavernosum. This may occur by restoring the endogenous amino acid pool for NO synthesis and by NO-cGMP-protein kinase G signaling involving the activation of Kca channels or by inhibiting the up-regulated RhoA/Rho-kinase pathway [Citation33].
In the CC strips from aged rats, endothelium-dependent relaxation to ACh was significantly reduced. Earlier studies supported our data [Citation30,Citation34,Citation35]. In pudendal artery from aged rats, relaxation to ACh was reduced [Citation6]. This finding suggests that endothelial layer of the CC from aged rats may be damaged. Therefore, the impairment in endothelial relaxation might result from decreased NO release and/or a direct action of the aging to endothelium. The eNOS is a key enzyme responsible for the release of NO from cavernosal endothelium. In this study, immunostaining for eNOS enzyme expression suggested that the molecular mechanism responsible for the diminished erectile response appears to involve the diminished eNOS levels in the CC of aged rats. It is well known that impaired endothelium-dependent relaxation of CC in aged rats might be due to changes in eNOS expression and/or activity. In a recent study by Dalaklioglu et al. [Citation36], both total eNOS protein expression and eNOS phosphorylation at Ser 1177 were decreased in CC of aged rats compared with that of control rats.
In the current study, we measured the levels of NOx metabolites (nitrite and nitrate, which indicate the production of NO) in the CC. We found that the cavernosal tissue NOx levels were significantly lower in the aged than in the control group. However, NaNO2 treatment resulted in an increase NOx concentration in aged penile tissue. In aged penile tissue obtained NaNO2 supplementation significantly increased NOx levels, but not altered eNOS expression and promoted erectile response in aged rats. Hence, the changes in penile NOx concentration may not be simply in response to the changes in the erectile response. Aging is normally associated with an increase in the level of oxidation. On the other hand, in our study, we observed the loss of smooth muscle fibers of male cavernous tissue that may lead to reduced oxygen tension in the penis. Changes in the ratio of penile collagen have also been observed and could explain the decrease in penile elasticity and compliance. This condition may compromise the oxygenation of the organ and downregulate local NO synthesis, a major neurotransmitter in erection [Citation37]. Moreover, NaNO2 may also lead to high levels of methemoglobin, leading to anemic hypoxia, a condition in which there is an inadequate supply of oxygen to tissues. Taken together, these effects may explain these aspects in the failure of NaNO2 treatment in penile tissue from aged rats.
In the previous study, Lasker and colleagues [Citation27] showed that the administration of NaNO2 increases erectile activity in the rat. While the authors administrated intracavernously the substance, we gave NaNO2 study in the drinking water. Although NO donors are widely used in cardiovascular medicine, with improvements in cholinergic transmission, relaxation of cardiomyocytes and dilatation of the coronary vessels, it simultaneously aggravates oxidative stress and induces death of cardiomyocytes.
In the current study, we showed that ACh-induced relaxation response in the CC of treated rats was not normalized by NaNO2 treatment. Our data may explain that endothelial damage in aging may not be recovered by endogen prolonged NO supplementation. However, relaxation responses of ACh in CC from aged and NaNO2-treated groups were partially reversed by the incubation of l-arginine or BH4. Previous data showed that long-term oral administration of l-arginine augmented erectile response in aged rats [Citation38]. Otherwise, l-citrulline as an amino acid plays a role as a donor for l-arginine/NO pathway of penile erection. It was shown that oral l-citrulline supplementation improved in erectile hardness with wild ED in men [Citation39] and acutely arteriogenic ED in rats [Citation40]. In addition, sepiapterin (a BH4 precursor) treatment developed erectile function in aged rats [Citation41]. In contrast, supplementation of substrate and co-factor in the short period may cause to some extent renovation. Other factors may have the evolving role in the regulation of the process. Perhaps the presence cofactor in the milieu can stimulate its activation because of the findings. In this study, endothelium-independent relaxation response to SNP was not altered in CC strips from all groups. Thus, the CC smooth muscle responses are at normal range in aged and treated rats. Similarly, the relaxation responses of SNP were not changed in aged rats in previous studies [Citation35]. In contrast, relaxation to SNP was decreased in CC from aged group in some studies [Citation30,Citation42]. An important observation was that the collagen deposition increased with age- and NaNO2-treated groups. Thereby, we except a unalteration in SNP-induced relaxation response due to the diminished amount of viable smooth muscle within the cavernosal tissues under aging. The difference is not clear based on our data in which SM/collagen ratio is reduced by endothelium-independent relaxation is unchanged. We suggest that intensified NO donor response may partially protect the smooth muscle from raised collagen deposition.
It is well known that sympathetic nervous system modifies penile flaccidity. In this study, the contractile response to Phe (α-1 adrenergic receptor agonist) was reduced in CC strips from aged rats. There are conflicting previous results regarding Phe responses in aged condition. In a study by Yousif et al. [Citation43], the contractile response to Phe was increased in aged CC. Non-adrenergic neurogenic contractile responses to EFS in the CC strips were reduced in aged rats when compared to control rats. In this study, we also presented that NaNO2 treatment did not correct Phe-induced and neurogenic contractile responses in aged rats. Conceivably noradrenergic nerve innervation in the CC from aged and NaNO2-treated groups was diminished by noradrenaline release in our data.
Conclusions
In conclusion, erectile response of CN was considerably reduced in aged rats, and NaNO2 treatment was not successful on ED in vivo studies. The EFS-induced relaxation in vitro bath condition was remarkable elevated while ACh-induced relaxation and eNOS enzyme expression were weakened. The relaxations to ACh and EFS were relatively ameliorated by the incubations of l-arginine and BH4. Accordingly aging process may reconcile impairment in bioavailability of NO or NO system in erectile tissue. NaNO2 as a storage form of NO is not advantageous while NOx levels were raised in aged penis. Future studies are warranted to explore the short and prolonged treatment of NO substrate and cofactor in aged circumstances.
Declaration of interest
The authors report no conflicts of interest.
References
- Corona G, Rastrelli G, Maseroli E, et al. Sexual function of the ageing male. Best Pract Res Clin Endocrinol Metab 2013;27:581–601
- Seftel AD. Erectile dysfunction in the elderly: epidemiology, etiology and approaches to treatment. J Urol 2003;169:1999–2007
- Rajasekaran M, Kasyan A, Jain A, et al. Altered growth factor expression in the aging penis: the Brown-Norway rat model. J Androl 2002;23:393–9
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54–61
- El-Sakka AI, Yassin AA. Amelioration of penile fibrosis: myth or reality. J Androl 2010;31:324–35
- Hannan JL, Blaser MC, Oldfield L, et al. Morphological and functional evidence for the contribution of the pudendal artery in aging-induced erectile dysfunction. J Sex Med 2010;7:3373–84
- Carrier S, Nagaraju P, Morgan DM, et al. Age decreases nitric oxide synthase-containing nerve fibers in the rat penis. J Urol 1997;157:1088–92
- Akarte AS, Srinivasan BP, Gandhi S. A novel long acting DPP-IV inhibitor PKF-275-055 stimulates beta-cell proliferation resulting in improved glucose homeostasis in diabetic rats. Biochem Pharmacol 2012;83:241–52
- Bakircioglu ME, Sievert KD, Nunes L, et al. Decreased trabecular smooth muscle and caveolin-1 expression in the penile tissue of aged rats. J Urol 2001;166:734–8
- Davila HH, Rajfer J, Gonzalez-Cadavid NF. Corporal veno-occlusive dysfunction in aging rats: evaluation by cavernosometry and cavernosography. Urology 2004;64:1261–6
- Ferrini M, Magee TR, Vernet D, et al. Aging-related expression of inducible nitric oxide synthase and markers of tissue damage in the rat penis. Biol Reprod 2001;64:974–82
- Ferrini MG, Davila HH, Valente EG, et al. Aging-related induction of inducible nitric oxide synthase is vasculo-protective to the arterial media. Cardiovasc Res 2004;61:796–805
- Pu XY, Wang XH, Gao WC, et al. Insulin-like growth factor-1 restores erectile function in aged rats: modulation the integrity of smooth muscle and nitric oxide-cyclic guanosine monophosphate signaling activity. J Sex Med 2008;5:1345–54
- Jin L, Liu T, Lagoda GA, et al. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J 2006;20:536–8
- Haas CA, Seftel AD, Razmjouei K, et al. Erectile dysfunction in aging: upregulation of endothelial nitric oxide synthase. Urology 1998;51:516–22
- Champion HC, Bivalacqua TJ, Hyman AL, et al. Gene transfer of endothelial nitric oxide synthase to the penis augments erectile responses in the aged rat. Proc Natl Acad Sci USA 1999;96:11648–52
- Bivalacqua TJ, Champion HC, Mehta YS, et al. Adenoviral gene transfer of endothelial nitric oxide synthase (eNOS) to the penis improves age-related erectile dysfunction in the rat. Int J Impot Res 2000;12:S8–17
- Musicki B, Kramer MF, Becker RE, Burnett AL. Age-related changes in phosphorylation of endothelial nitric oxide synthase in the rat penis. J Sex Med 2005;2:347–55; discussion 355–7
- Bivalacqua TJ, Burnett AL, Hellstrom WJ, Champion HC. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am J Physiol Heart Circ Physiol 2007;292:H1340–51
- Musicki B, Champion HC, Becker RE, et al. Erection capability is potentiated by long-term sildenafil treatment: role of blood flow-induced endothelial nitric-oxide synthase phosphorylation. Mol Pharmacol 2005;68:226–32
- Hedlund P, Aszodi A, Pfeifer A, et al. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc Natl Acad Sci USA 2000;97:2349–54
- Bivalacqua TJ, Armstrong JS, Biggerstaff J, et al. Gene transfer of extracellular SOD to the penis reduces and improves erectile function in aged rats. Am J Physiol Heart Circ Physiol 2003;284:H1408–21
- Tomada I, Tomada N, Almeida H, Neves D. Androgen depletion in humans leads to cavernous tissue reorganization and upregulation of Sirt1-eNOS axis. Age (Dordr) 2013;35:35–47
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–76
- Tomada N, Tomada I, Cruz F, et al. Characterization of VEGF and angiopoietins expression in human corpus cavernosum during aging. J Sex Med 2010;7:1410–8
- Cordeiro AL, Figueiredo A, Tomada I, et al. Characterization of the expression of Ang1, Ang2, and Tie2 in the Corpus Cavernosum of the rat during aging. Microsc Microanal 2010;16:699–709
- Lasker GF, Matt CJ, Badejo AM Jr, et al. Intracavernosal administration of sodium nitrite as an erectile pharmacotherapy. Can J Physiol Pharmacol 2010;88:770–6
- Oztekin CV, Gur S, Abdulkadir NA, et al. Incomplete recovery of erectile function in rat after discontinuation of dual 5-alpha reductase inhibitor therapy. J Sex Med 2012;9:1773–81
- Pinsky MR, Gur S, Tracey AJ, et al. The effects of chronic 5-alpha-reductase inhibitor (dutasteride) treatment on rat erectile function. J Sex Med 2011;8:3066–74
- Silva FH, Monica FZ, Bau FR, et al. Superoxide anion production by NADPH oxidase plays a major role in erectile dysfunction in middle-aged rats: prevention by antioxidant therapy. J Sex Med 2013;10:960–71
- Nunes KP, Toque HA, Borges MH, et al. Erectile function is improved in aged rats by PnTx2-6, a toxin from Phoneutria nigriventer spider venom. J Sex Med 2012;9:2574–81
- Angulo J, Cuevas P, Fernandez A, et al. Activation and potentiation of the NO/cGMP pathway by NG-hydroxyl-L-arginine in rabbit corpus cavernosum under normoxic and hypoxic conditions and ageing. Br J Pharmacol 2003;138:63–70
- Gur S, Kadowitz PJ, Trost L, Hellstrom WJ. Optimizing nitric oxide production by time dependent L-arginine administration in isolated human corpus cavernosum. J Urol 2007;178:1543–8
- Sindler AL, Fleenor BS, Calvert JW, et al. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 2011;10:429–37
- Cartledge JJ, Eardley I, Morrison JF. Nitric oxide-mediated corpus cavernosal smooth muscle relaxation is impaired in ageing and diabetes. BJU Int 2001;87:394–401
- Dalaklioglu S, Sahin P, Tasatargil A, Celik-Ozenci C. Pravastatin improves the impaired nitric oxide-mediated neurogenic and endothelium-dependent relaxation of corpus cavernosum in aged rats. Aging Male 2013. [Epub ahead of print]
- Wespes E. Smooth muscle pathology and erectile dysfunction. Int J Impot Res 2002;14:S17–21
- Moody JA, Vernet D, Laidlaw S, et al. Effects of long-term oral administration of L-arginine on the rat erectile response. J Urol 1997;158:942–7
- Cormio L, De Siati M, Lorusso F, et al. Oral L-citrulline supplementation improves erection hardness in men with mild erectile dysfunction. Urology 2011;77:119–22
- Shiota A, Hotta Y, Kataoka T, et al. Oral L-citrulline supplementation improves erectile function in rats with acute arteriogenic erectile dysfunction. J Sex Med 2013;10:2423–9
- Johnson JM, Bivalacqua TJ, Lagoda GA, et al. eNOS-uncoupling in age-related erectile dysfunction. Int J Impot Res 2011;23:43–8
- Yousif MH, Kehinde EO, Benter IF. Different responses to angiotensin-(1-7) in young, aged and diabetic rabbit corpus cavernosum. Pharmacol Res 2007;56:209–16
- Yousif MH, Benter IF. Role of cytochrome P450 metabolites of arachidonic acid in regulation of corporal smooth muscle tone in diabetic and older rats. Vascul Pharmacol 2007;47:281–7