Abstract
Objective: Prediabetes patients are likely to develop type 2 diabetes (T2DM). Low testosterone is a risk factor for impaired glucose tolerance (IGT) in men. The aim of this study was to investigate the prevalence of prediabetes in population of Polish men with late-onset hypogonadism (LOH).
Methods: This study was performed in 246 men with LOH and in 184 eugonadal control group. Prediabetes was diagnosed in patients with impaired fasting glucose (IFG), IGT or with HbA1c from 5.7 to 6.4%. Sex hormones and metabolic parameters were measured.
Results: The mean TT concentration in the LOH group was 9.55 ± 1.5 nmol/l and 16.45 ± 1.8 nmol/l in the control group (p < 0.001). We observed negative relationships between cFT and HbA1c (r = −0.336; p < 0.005) and between TT and HbA1c (r = −0.366, p < 0.002), In the LOH group, prediabetes was diagnosed in 41.5% men. In the control group, prediabetes was diagnosed in 13% of patients. In the LOH group, TT and cFT levels were lower in prediabetic patients, when compared with normoglycemic patients and patients with IGT had lower TT levels than subgroups with IFG or elevated HbA1c.
Conclusions: In a population of Polish men with LOH, we observed high prevalence of prediabetes and routine fasting glucose and glucose tolerance test should be performed in these patients.
Introduction
Male aging is associated with a decline in serum total testosterone (TT) approximately 1–2% per year in cohorts of men followed for 7–10 years in the Massachusetts Male Aging Study (MMAS) [Citation1]. Low testosterone is also an independent risk factor for high-fasting plasma glucose [Citation2] leading to an increased prevalence of impaired glucose tolerance (IGT) and type 2 diabetes mellitus (T2DM) in men [Citation3]. Approximately 7% of the world population is estimated to have IGT. The vast majority of these people live in low- and middle-income countries. By the year 2035, the number of people with IGT is projected to increase to 8.0%. But in Polish population, the prevalence of IFG in 2013 is 16.5% and is estimated to increase to 19.3% by the year 2035, so it will be the highest in the world [Citation4].
A causal role for testosterone in the relationship between insulin resistance, metabolic syndrome (MetS) and T2DM in men is suggested by epidemiological data in men with and without T2DM [Citation5,Citation6]. TT levels have been studied in populations of men with T2DM with estimates that 30–50% of men with T2DM have low testosterone [Citation7]. One of the strongest correlates with TT in aging men is obesity [Citation8] with a 30% reduction of TT levels in obese men in MMAS cohort [Citation9]. In the European Male Aging Study (EMAS), obesity was the most important predictor of low TT [Citation10]. In the Hypogonadism in Males Study (HIM) [Citation11], 33% of lean, 44% of overweight and 46% of obese T2DM men aged >45 years had TT levels below 10.5 nmol/l. The longitudinal studies have demonstrated that men with low TT levels are at a greater risk of future development of T2DM [Citation5,Citation6], but the mechanisms of this impact are not fully understood and current studies suggest that androgens may influence on insulin sensitivity and prevalence of obesity and MetS [Citation6,Citation7].
Prediabetes is the condition in which the people have slight increase in blood glucose levels than the normal levels but they are not said to be diabetic. The American Diabetes Association (ADA) defines prediabetes as IFG, IGT and glycated hemoglobin (HbA1c) of 5.7–6.4% [Citation12]. Prediabetes patients are likely to develop T2DM within 10 years, unless they take steps to prevent or delay diabetes. They are also in high risk to develop cardiovascular complications [Citation13].
Data from non-diabetic men have revealed an inverse association between insulin resistance and testosterone concentrations [Citation7,Citation8]. Prediabetes is characterized by an increased insulin resistance and that is why interesting, whether prediabetes is associated with low testosterone levels and what is prevalence of prediabetes among patients with testosterone deficiency, especially in population of men with multiple comorbidities, as observed in Poland.
Only few studies have demonstrated relationships between androgens and prediabetes in men [Citation14]; thus, the aim of this study was to investigate the prevalence of prediabetes in a population of Polish men with late-onset hypogonadism (LOH) and possible relationships with comorbidities.
Materials and methods
This study was performed in 246 men, aged 47–76 years, with signs and symptoms of LOH and in 184 eugonadal control group. The study was performed in the Department of Internal Diseases, Diabetology and Endocrinology, Warsaw Medical University and Department of Endocrinology, Medical Centre for Postgraduate Education in Warsaw, Poland. All patients gave written informed consent and the local research ethics committee approved the protocol. We excluded patients with obvious pre-existing renal and liver failure, insufficiency of hypophysis and primary hypogonadism, impaired thyroid gland function and hyperprolactinemia. We also excluded those who received testosterone replacement therapy or received androgen deprivation therapy for prostate cancer. Demographic parameters, clinical history including the duration of diabetes, medications and the presence of erectile dysfunction and cardiovascular disease (CVD) were collected and height, weight, fasting plasma glucose (FPG), oral glucose tolerance test (OGTT) (in patients with no history of diabetes) and HbA1c were measured.
LOH was diagnosed in patients with signs and symptoms of testosterone deficiency like: low libido, diminished frequency of morning erections and erectile dysfunctions. One or more of these symptoms must be corroborated with a decreased serum TT levels below 12 nmol/l (350 g/dl) or cFT levels <0.25 nmol/l [Citation15].
T2DM was diagnosed in 25 patients if the patient had a prior history of diabetes or if the glycemic variables reached the criteria of diabetes: fasting glucose ≥126 mg/dl or two-hour postprandial glucose ≥200 mg/dl [Citation12] and five diabetic patients were treated with diet alone, eight patients with oral hypoglycemic agents and 12 with insulin.
According to recommendations of ADA [Citation12], prediabetes was diagnosed in patients with impaired fasting glucose from 100 to 125 mg/dl and two-hours glucose concentration in OGTT <140 mg/dl or in patients with impaired glucose tolerance – two-hour glucose concentration in OGTT from 140 to 200 mg/dl or in patients with HbA1c 5.7–6.4%.
The diagnosis of metabolic syndrome (MetS) was based on the IDF definition 2005 [Citation16]. To diagnose MetS must be fulfilled the following criteria: waist circumference ≥94 cm, and any two of the following: triglycerides ≥150 mg/dl, HDL-cholesterol <40 mg/dl, blood pressure ≥130/85 mmHg and FPG ≥110 mg/dl. Height, weight and waist circumference were measured and body mass index (BMI) was calculated. Obesity was defined as a body-mass index (the weight in kilograms divided by the square of the height in meters) of 30 or more. CVD was defined as self-reported coronary artery disease, congestive heart failure or arrhythmia. Hypertension and hyperlipidemia were also considered to be present if the participant reported having received the diagnosis or if he was receiving medication for the condition.
Fasting blood samples were then obtained between 8:00 and 10:00 A.M. to measure serum TT, estradiol (E2), sex hormone binding globuline (SHBG), luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin (PRL), fasting plasma glucose (FPG) and HbA1c. All men had their total testosterone (TT), LH, FSH and PRL levels checked at least once. TT, LH, FSH, SHBG, DHEA and PRL were measured by chemiluminescent immunometric assays (Immulite 2000; DPC United States and Coat-a-Coat; Siemens, Los Angeles, CA). The normal value for testosterone was 8–28 nmol/l, for LH: 2–6 mIU/l, for FSH: 3–10 mIU/l, for PRL: 12–24 ng/ml and for DHEA-S 200–375 ng/dl. Calculated free testosterone (cFT) was calculated from SHBG, serum albumin and TT using the method of Vermeulen and colleagues [Citation17] – cFT level <0.255 nmol/l was taken as low.
Statistical analysis was performed using Statistica software (Statistica 10, StatSoft Inc, USA). Data are presented as mean ± SD and categorical data are presented as count and percentage (%). To establish correlation, Spearman's test was used to compare non-parametric data and Pearson's test to compare parametric data. All relationships were assessed by linear univariate and multivariate regression analysis to reduce bias in a cross-sectional study. In multivariate analysis, statistical data were adjusted for age, BMI and MetS. Results were considered statistically significant at p < 0.05.
Results
A total of 246 men, aged 47–76 years (mean age 67.3 ± 3.2 years), with LOH and 184 eugonadal control men, aged 51–77 years (mean age 65.8 ± 3.4 years) were evaluated. Characteristics of both groups are shown in . The mean TT concentration in the LOH group was 9.55 ± 1.5 nmol/l and 16.45 ± 1.8 nmol/l in the control group (p < 0.001), cFT levels were 0.323 ± 0.07 nmol/l and 0.386 ± 0.07 nmol/l, respectively (p < 0.001). We observed significantly higher E2 levels in the LOH group (p < 0.002), LH levels were also significantly higher in the LOH group (p < 0.02) but FSH, DHEA and prolactin concentrations did not differ significantly between groups. BMI and waist circumference were statistically significantly higher in the LOH group (p < 0.02 and p < 0.05, respectively). Differences of systolic and diastolic pressure were not significant.
Table 1. Characteristic of patients with LOH and control group.
In the LOH group, we have shown significant negative relationships between BMI and TT (r = −0.3723; p < 0.01) and between BMI and cFT (r = −0.352, p < 0.01) but there were no significant correlations between waist circumference and TT or cFT in the LOH group. We have also observed significant negative relationships between cFT and HbA1c (r = −0.336; p < 0.005) and between cFT and triglicerydes (r = −0,355, p < 0.002) but TT levels were correlated significantly only with HbA1c (r = −0.366, p < 0.002). In this multivariate analysis, these relationships were significant after adjustment for age, BMI and MetS.
There were significant differences of metabolic and glycemic control parameters between both groups. In the LOH group, T2DM was diagnosed in 25 patients (10.1%), prediabetes in 102 patients (41.5%) and 120 men (48.4) were normoglycemic. In prediabertic patients, IFG was diagnosed in 60 men (24.4% of all LOH group and 50% of prediabetic patients) and IGT in 56 patients (22.8% of LOH group and 48.6% of prediabetic patients). In 88 (86%) prediabetic men in the LOH group HbA1c in range from 5.7 to 6.4% was found. In additio, four men without IGF or IGT, HbA1c was from 5.7 to 6.4% and they were also classified as prediabetic patients – .
Figure 1. The prevalence (%) of normoglycemia, prediabetes and T2DM in patients with LOH and in control group. T2DM – diabetes mellitus type 2.
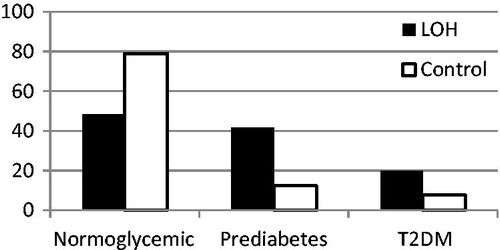
In 184 control group, T2DM was diagnosed in 14 patients (7.6%), prediabetes in 24 patients (13%) and 146 (79.4%) of them were normoglycemic. In prediabetic patients, IGF was diagnosed in 14 men (7.5% of eugonadal group and 56% of prediabetic patients) and IGT in 10 patients (5.3% of eugonadal group and 44% of prediabetic patients). From prediabetic patients in seven (28%) HbA1c was from 5.7 to 6.4%. The mean HbA1c in both group are presented in .
Mean TT and cFT levels among patients prediabetic, normoglycemic and diabetic patients in the LOH group are presented in . TT and cFT levels were significantly lower in patients with prediabetes when compared with normoglycemic patients in the LOH group (p < 0.02 and p < 0.002, respectively). Additional statistical analysis of the LOH group revealed that patients with IGT have significantly lower TT levels than all prediabetic group and subgroups with IFG and elevated HbA1c. In this analysis, cFT levels did not differ between prediabetic patients. In this multivariate analysis, these relationships were significant after adjustment for age, BMI and MetS.
Table 2. Total and free testosterone concentrations in the LOH group after dividing according to glycemic control disorders.
In the LOH group, obesity was diagnosed in 57% patients, while diagnosis of MetS was established in 66% and differences with control group were significant (p < 0.002 and 0.02; respectively) – . The prevalence of CVD was significantly higher in the LOH group but prevalence of hypertension and smoking did not differ between groups.
Table 3. Comorbidities (no.pts.;%) in all LOH patients, prediabetic and normoglycemic subgroup and in the control group.
Discussion
In our study, we evaluated the prevalence of prediabetes in the population of 246 men above 50 years with LOH and it was the first study, which was performed in a relatively large population of men in Eastern Europe. As far as we know, this is also the first report showing such high prevalence of prediabetes in men with LOH and poor health status.
We demonstrated that in this cohort of men with LOH, prediabetes was diagnosed in 41.5% and only in 13% in control, eugonadal group in the same age. We also showed significantly higher prevalence of T2DM in the LOH group (10.1 and 7.6%, respectively). In patients with LOH, there were negative relationships between TT and HbA1c, cFT and HbA1c and also cFT and triglycerides. These relationships were still significant after adjustment of age, BMI and MetS.
In our study, prediabetes was diagnosed according to the recommendations of ADA in patients with IFG, IGT and HbA1c; 5.7–6.4% [Citation12]. Currently, an intermediate HbA1c range is considered as prediabetes by the ADA, but not by the WHO. However, prediabetes identified by IFG, IGT or an intermediate HbA1c range may be caused by different mechanisms and as well as different population [Citation18]. We also suggest that such an identification gives wider range of subjects at the risk group of T2DM development. In our study in prediabetes subgroup of LOH patients, we observed IFG in 50%, IGT in 48.6% and elevated HbA1c in 1.4% patients (without IFG or IGT) but 86% patients in all prediabetes group had elevated HbA1c.
In our cohort we observed that mean TT and cFT levels were significantly lower in patients with prediabetes when compared with normoglycemic patients in the LOH group, while further analysis revealed, that patients with IGT had significantly lower TT levels than all prediabetic group and subgroup with IFG and these relationships were still significant after adjustment for obesity and MetS. These results indicate that low testosterone concentration is an independent risk factor of prediabetes and probably may affect rather on glucose tolerance in standard OGTT than on fasting plasma glucose concentration. Regardless of these details associated with diagnosis, prediabetes in patients with LOH is very common, independent from obesity and metabolic syndrome and should be taken under consideration during evaluation of patients with signs and symptoms of hypogonadism, especially in Polish population.
The prevalence of T2DM and prediabetes is rapidly increasing but in Poland is higher than it is observed in other countries. In the general Polish population aged 20–74 years, diabetes was diagnosed in 7.4% men but the prevalence of IFG is the highest in the world and is estimated to reach 19.3% of population by the year 2035 [Citation4]. T2DM in men is associated with lower testosterone levels in cross-sectional studies and the majority of these men have signs and symptoms of hypogonadism, such as erectile dysfunctions, low libido, fatigue, sarcopenia and depression [Citation3]. In our previous study, performed in Polish population, we documented, that in 184 T2DM men, mean age 58.5 ± 2.3 years, 46% patients were hypogonadal and 93% had hypogonadotropic hypogonadism. We observed significant inverse relationship between TT and BMI (r = −0.362, p < 0.01) and between HbA1c and TT levels (r = −0.346, p < 0.002). So, we showed high prevalence of hypogonadism among men with T2DM in Polish population, which was associated with poor glycemic control [Citation19].
Only few studies have demonstrated relationships between androgens and prediabetes in men, Colangelo et al. [Citation20] in Multi-Ethnic Study of Atherosclerosis (MEST) observed negative relationship between testosterone and T2DM and IFG in 3156 men and these relationships were significant after adjustment for age and BMI. Also Ho et al. [Citation14], in 1306 men, showed that prediabetes is associated with an increased risk of testosterone deficiency, independent of obesity and MetS. In the Rancho Bernardo Study [Citation21], sex hormones levels were evaluated in 775 men and in patients with IFG or IGT, TT levels were lower than in men with normal glucose tolerance. Corona et al. [Citation22] evaluated the impact of IFG on sexual health in 3451 men and found that IFG increases the risk of testosterone deficiency. These cross-sectional studies were performed in large populations of healthy men or in men with sexual dysfunctions, but with normal testosterone levels. Our study was performed only in patients with low testosterone levels (<12 nmol/l), in population with high prevalence of co-existing comorbidities.
The possible pathophysiological mechanisms causing prediabetes in testosterone deficiency men are still unknown. Low testosterone levels in men are associated with insulin resistance and reduced insulin sensitivity [Citation7]. Moreover, low testosterone levels have also been found to predict insulin resistance, obesity and T2DM [Citation23]. Testosterone can be converted to estradiol in the adipose tissue and it has been suggested that excessive estrogen secretion in the obese may suppress secretion of testosterone [Citation24]. In our study, estradiol concentration was significantly higher in men with LOH and prediabetes.
Low testosterone concentration in patients with T2DM is strongly associated with elevated risk of cardiovascular events and death [Citation25,Citation26]; therefore, possible hypogonadism should be diagnosed in men with prediabetes and T2DM [Citation15]. On the other hand, in men with LOH disturbances of glucose metabolism are common, and fasting plasma glucose and OGTT should be measured.
In a previous study, the prevalence of testosterone deficiency was not dependent on severity of hyperglycemia assessed as glycosylated hemoglobin (HbA1c) levels [Citation27]. In our study, we observed significant negative correlation between HbA1c and testosterone concentration, thus in the population of Polish men with LOH severity of testosterone deficiency can be associated with the high prevalence of prediabetes. These findings can also be related to relative late identification of prediabetes as well as LOH in Polish population (mean HbA1c level in LOH group was 6.1% and 5.4% in control group; p < 0.02).
The incidence of hypogonadism among patients with T2DM was significantly greater in our study than observed in studies cited above. Hypogonadism can be associated with diabetes per se, but vascular factors, drugs, tobacco, alcohol and systemic diseases such as hypertension, heart diseases and dyslipidemia can be risk factors of testosterone deficiency. In our study, we observed high prevalence of comorbidities (over 50% for each condition). High prevalence of comobidities in our cohort, higher than in cited studies, can be one of the explanations of high prevalence of prediabetes in patients with LOH in Poland. However, associations of TT levels with prediabetes prevalence, as well as HbA1c, were significant after adjustment for obesity and MetS, strong risk factors of prediabetes and T2DM.
Testosterone deficiency is associated with the risk of metabolic diseases, vascular complications of diabetes [Citation28], risk for developing T2DM and mortality. So testosterone replacement therapy may be taken into account in patients with absence of modifiable etiology of hypogonadism and contraindications to treatment [Citation15]. Short-term studies in men have shown that testosterone supplementation may improve insulin sensitivity [Citation29,Citation30] and survival in hypogonadal men with T2DM [Citation31]. Also long-term studies [Citation32–34] demonstrated that testosterone therapy for up to 6 years resulted in significant and sustained improvements in glycemic control and other cardio-metabolic risk factors in diabetic men with testosterone deficiency.
Our model of study in no way established a causal link between prediabetes and testosterone deficiency, because these conditions might simply overlap and they may have probably separate pathophysiologic pathways. The most widely accepted parameter to establish the presence of hypogonadism is the measurement of TT. Unfortunately, no consensus has been reached regarding the lower TT threshold defining LOH, and there are no generally accepted lower limits of normal TT [Citation15]. In our study, we have chosen 12 nmol/l as a cut-off for low limits testosterone of normal range because the prevalence of LOH symptoms increases with TT levels below 12 nmol/l (350 ng/dl) [Citation35].
In conclusion, in this population of Polish men with late-onset hypogonadism, we observed high prevalence of prediabetes and routine fasting glucose, glucose tolerance test and HbA1c should be performed in all hypogonadal men to confirm or exclude prediabetes.
Declarations of interest
The authors report no conflicts of interest.
This study was supported by research grant number 501-2-1-07-21/09 of the Medical Centre for Postgraduate Education, Poland.
References
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2002;87:589–98
- Mazur A, Westerman R, Werdecker A, Mueller U. Testosterone and type 2 diabetes in men. Aging Male 2014;1:18–22
- Kim ML, Rolland O, Cepeda JK, et al. Diabetes mellitus in older men. The Aging Male 2006;9:139–47
- Cho NH, Whiting D, Guariguata L, et al. IDF Diabetes Atlas. 6th ed. International Diabetes Federation; 2013:39–49
- Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004;27:1036–41
- Stellato RK, Feldman HA, Hamdy O, et al. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care 2000;23:490–4
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008;93:1834–40
- Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes 2010;17:224–32
- Field AE, Colditz GA, Willett WC, et al. The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone-binding globulin in middle aged men. J Clin Endocrinol Metab 1994;79:1310–16
- Tajar A, Huhtaniemi IT, O'Neill TW, et al; EMAS Group. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab 2012;5:1508–16
- Dhindsa S, Miller MG, McWhirter CL,et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010;33:1186–92
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35:1–8
- Rydén L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2013;34:3035–87
- Ho CH, Yu HJ, Wang CY, et al. Prediabetes is associated with an increased risk of testosterone deficiency, independent of obesity and metabolic syndrome. PLos One 2013;8:e74173
- Lunenfeld B, Mskhalaya G, Kalinchenko S, Tischova T. Recommendations on the diagnosis, treatment and monitoring of late-onset hypogonadism in men – a suggested update. Aging Male 2013;4:143–50
- Athyros GV, Ganotakis ES, Mikhailidis DO. The prevalence of the metabolic syndrome using the National Cholesterol Educational Program and International Diabetes Federation definitions. Curr Med Res Opin 2005;21:1157–64
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84 3666–72
- Saukkonen T, Cederberg H, Jokelainen J, et al. Limited overlap between intermediate hyperglycemia as defined by A1C. pp. 5.7–6.4%, impaired fasting glucose, and impaired glucose tolerance. Diabetes Care 2011;34:2314–16
- Rabijewski M. Papierska L, Zgliczyński W, Piątkiewicz P. The Incidence of hypogonadotropic hypogonadism in type 2 diabetic men in Polish population. BioMed Res Int 2013;2013:767496
- Colangelo LA, Ouyang P, Liu K, et al. Association of endogenous sex hormones with diabetes and impaired fasting glucose in men: multi-ethnic study of atherosclerosis. Diabetes Care 2009;32:1049–51
- Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care 2000;23:912–18
- Corona G, Rastrelli G, Balercia G, et al. Hormonal association and sexual dysfunction in patients with impaired fasting glucose: a cross-sectional and longitudinal study. J Sex Med 2012;9:1669–80
- Salving E, Finley M, Zhang L, et al. Androgens and diabetes in men. Results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care 2007;30:234–8
- Piteous N, Dwyer AA, Decius S, et al. The relative role of gonad sex steroids and gonadotropin-releasing hormone pulse frequency in the regulation of follicle-stimulating hormone secretion in men. J Clin Endocrinol Metab 2008;93:2686–92
- Laughlin GS, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 2008;93:68–75
- Muraleedharan V, Marsh H, Kapoor D, et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol 2013;6:725–33
- Kapoor D, Aldred H, Clark S, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007;30:911–17
- Phillips OB, Jing T, Heymsfield SB. Relationships in men of sex hormones, insulin, adiposity, and risk factors for myocardial infarction. Metabolism 2003;52:784–90
- Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 2011;34:828–37
- Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl 2009;30:726–33
- Muraleedharan V, Marsh H, Kapoor D, et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol 2013;169:725–33
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010;7:3495–503
- Francomano D, Lenzi A, Aversa A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol 2014. (Epub ahead of print)
- Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with “diabesity”: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol 2014. (Epub ahead of print)
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab 2006;91:4335–43
