Abstract
Late-onset hypogonadism (LOH) is a clinical syndrome characterized with aging and declined serum testosterone levels. Sexual symptoms are also essential for the diagnosis of LOH. Testosterone replacement therapy is used widely to treat LOH. However, the side effects of it should not be ignored, such as fluid retention, hypertension and spermatogenic suppression. Therefore, alternate treatment modalities have been pursued. Herbal medicines used widely in China have achieved satisfying results with little side effects. Nonetheless, there are few pharmacological researches on them. In this study, 24-month-old mice were used as LOH animal models to explore the pharmacological effects of a herbal medicine, saikokaryukotsuboreito (SKRBT), on serum testosterone levels and sexual functions. Furthermore, the expression of steroidogenic acute regulatory (StAR) protein, a kind of rate-limiting enzyme of testosterone synthesis, was also examined. As a result, SKRBT improved the serum testosterone levels of these mice at a dose of 300 and 450 mg/kg. Multiple measures of sexual behavior were enhanced. The expression of StAR was also increased. Therefore, this study suggested that SKRBT can improve the serum testosterone levels by activating the expression of StAR and might be a viable option to treat sexual symptoms caused by LOH.
Introduction
It has been estimated that the number of individuals over 65 years old has increased more than 10-fold in the past 100 years [Citation1]. And this phenomenon has boosted the development of geratology. Multiple organ systems undergo degenerative changes along with age. Many factors can affect these procedures, such as genetics, some diseases and the accumulated effects of socioeconomic, lifestyle, environmental factors [Citation2]. All physiological functions gradually decline along with age. For example, fat mass increase, muscle mass loss, bone mineral density decrease, fatigue, insomnia, poor concentration, depression and sexual dysfunction. Three sexual symptoms (lessened sexual thoughts, weakened morning erections and erectile dysfunction) are main symptoms of late-onset hypogonadism (LOH) and are crucial for the diagnosis of LOH [Citation3]. Sexual hormone levels decline is thought to be related to these changes. Several names have been used to describe the syndrome combining testosterone deficiency and the symptoms depicted above, including male menopause, partial androgen deficiency of the aging male and andropause. LOH was first used by Morales and Lunenfeld in 2002 [Citation4] and officially adopted by International Society of Andrology (ISA), International Society for the Study of the Aging Male (ISSAM), European Association of Urology (EAU) in 2005 [Citation5]. It is considered that LOH could best describe the nature of the syndrome and has been defined as “a clinical and biochemical syndrome associated with advancing age and characterized by symptoms and a deficiency in serum testosterone levels (below the young healthy adult male reference range)” [Citation6]. Leydig cell is the main source of testosterone in vivo. The total number of Leydig cells of the aged man decreases to around half of that seen in the testes of the young man [Citation7]. It has been found that the serum concentration of total testosterone begin to decline steadily at 30–40 years of age at a rate of about 1% every year [Citation8,Citation9], and result in 20% of men over 60 years and 50% of men over 80 years with lower serum testosterone than expected [Citation10]. Testosterone replacement therapy (TRT) is considered to be an effective therapy to relieve the main symptoms of LOH and improve the quality of life. Numerous placebo controlled trials have reported the benefits associated with TRT [Citation11–13]. Meanwhile, the contraindications and potential adverse effects of it could not be ignored. Prostate and breast cancer is the absolute contraindications for testosterone treatment. Relative contraindications include a serum PSA level >4 ng/ml, or >3 ng/mlin men with an increased risk for prostate cancer (e.g. African-Americans, first-degree relatives of men with prostate cancer), polycythemia (hematocrit >50%), severe lower urinary tract symptoms caused by benign prostatic hypertrophy (International Prostate Symptom Score >19), poorly controlled heart failure and untreated obstructive sleep apnea [Citation14]. It is well known that exogenous testosterone can impress fertility, so those who have the desire to start a family in the near future should avoid TRT [Citation15]. Searching a method to get the benefit from TRT while refraining from those adverse effects would be much significant.
Saikokaryukotsuboreito (SKRBT, Chai-Hu-Chia-Lung-Ku-Mu-Li-Tang in Chinese), one of the herbal medicines, comes from Treatise on Febrile Diseases, an ancient Chinese medical book written by Zhong Jing Zhang about 1800 years ago. According to that book, SKRBT should be used in treating neuropsychiatric disorders such as depression, palpitation, neurosis, anxiety, vertigo and insomnia. Most of which have been testified by clinical tests or animal experiments [Citation16–18]. Several clinical articles have reported that SKRBT is effective in treating LOH-related symptoms [Citation19,Citation20]. However, as far as we know, few articles on sexual behavior changes affected by SKRBT in LOH are reported till now.
In this study, 24-month-old mice were used as LOH animal models to examine the effects of SKRBT on the serum testosterone levels and sexual behavior. Moreover, we attempted to elucidate its effect on testosterone synthesis by testing the expression of steroidogenic acute regulatory (StAR) protein in testis because it is a rate-limiting enzyme of this process.
Materials and methods
Animals
Male and female C57BL/6 mice were purchased from the Animal Center of Sun Yat-sen University (Guangzhou, China). Males were approximately 24 months of age and females were about 3 months old. All animal studies were carried out in accordance with the guidelines of the Sun Yat-sen University Institutional Animal Care and Use Committee. Males were housed one per cage and females were housed five per cage. All mice were maintained on a reversed 12-h light-dark cycle, with lights on from 1900 to 0700 h, under the temperature (22 ± 1 °C), humidity (60%).
In behavioral testing, the female mice were used as stimulus females and were subjected to bilateral ovariectomy. One month later, these female mice were pretreated with estradiol benzoate (50 µg, 48 h before testing, dissolved in 50 µl of peanut oil) and progesterone (500 µg, 5 h before testing, dissolved in 50 µl of peanut oil) to reach a state of estrous.
The blood specimen of each male mouse was collected from the retrobulbar space and was checked the concentration of serum testosterone before the test. Only the male mice with the serum testosterone concentration below 10 ng/ml were selected and randomly assigned into four different groups. Each group contained 12 male mice. One group was treated with phosphate-buffered solution (PBS) as normal control. The other three groups were treated with SKRBT at different concentrations (150, 300 and 450 mg/kg).
Drugs
SKRBT is composed by 10 crude drugs in fixed proportions (), including Bupleurum root, Pinellia tuber, Cinnamon bark, Poria sclerotium, Scutellaria root, Jujube, Oyster shell, Ginseng, Longgu, Rhubarb and Ginger. The dried extract powder of SKRBT was manufactured in the GMP pharmaceutical factory of Kracie Pharma Ltd. The powdered drug was suspended in distilled water. Different dosage of SKRBT (150, 300 or 450 mg/10 ml/kg, p.o.) was administered daily for three weeks.
Table 1. Crude drug composition of SKRBT.
Testosterone assays
The serum was collected from the male mice before and after the test for the quantitative determination of testosterone. Testosterone levels were measured using a commercially available enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions.
Tests of sexual behavior
Tests were conducted in a clean testing cage (40 × 26 × 21 cm). The room was soundproof. Mounts, intromissions and ejaculations were recorded as sexual behaviors [Citation21]. The term “intromission” refers to a behavioral pattern consisting of repeated deep pelvic thrusting, generally associated with penile insertion. The term “ejaculation” refers to the behavior of ejaculation, which is characterized by the reflexive grasping of the female occurs at the end of an intromission, and often followed by the male falling off the female to the side [Citation22].
Several measure targets were scored and depicted as follows: mount latency, the time from introduction of the first female to the occurrence of the first mount; intromission latency, the time from introduction of the first female to the occurrence of the first intromission; ejaculation latency, the duration from the first intromission to ejaculation; frequency of mount, the number of mounts until ejaculation or until the test was terminated; frequency of intromission, the number of intromissions until ejaculation or until the test was terminated; copulatory efficacy: a measure of intromission success, calculated as intromission frequency divided by mount frequency + intromission frequency [Citation21].
Male mice were placed individually into the testing cage. Fifteen minutes later, a pretreated female mouse was placed into the testing cage with the pre-adapted male mouse. If 10 min passed without an intromission, the first female mouse was replaced with another hormone-stimulus female mouse. If the male mouse failed to achieve intromission with the second female mouse within the next 5 min, a third pretreated female mouse was introduced for a final 15 min period. Failure to achieve intromission within 15 min and ejaculation within 45 min of the start of the test, resulted intermination of the test, and the maximum latency value of 45 min was assigned for that behavior [Citation23]. All the sexual behavior tests were analyzed by an observer who did not know which group these mice belonged to.
Immunofluorescence staining
The frozen testis sections were blocked by incubation in 5% normal serum and 0.1% Triton X-100 (Hyclone) in PBS for 1 h at room temperature, followed by incubation with primary antibody overnight at 4 °C. After that the sections were incubated with secondary antibody for 30 min followed by 4′,6-diamidino-2-phenylindole (DAPI) (blue) staining of nuclei. The primary and secondary antibodies used are listed in . Negative controls were prepared by changing the primary antibody with PBS. All the images were captured using an OLYMPUS IX71 inverted research microscope and were analyzed by DP Manager version 3.1.1.208. The method of cell counting was similar to that employed in earlier study [Citation24]: two sections per testis, five testes per group, were analyzed. Random interstitial spaces defined as the space enclosed by three or four contorted seminiferous tubules were counted for the number of positive cells. The numbers of positive cells per group were averaged for statistical analysis. All counting was performed on an Olympus microscope (Center Valley, PA, magnification: 400×).
Table 2. Primary and secondary antibodies used for immunofluorescence analysis.
Statistical analysis
All the data were analyzed using one-way analysis of variance first. Individual between-group comparisons of changes in the serum testosterone levels were made using least significant difference t-test. Results are presented as mean ± SEM. In all cases, probability values of p below 0.05 were considered to be statistically significant.
Results
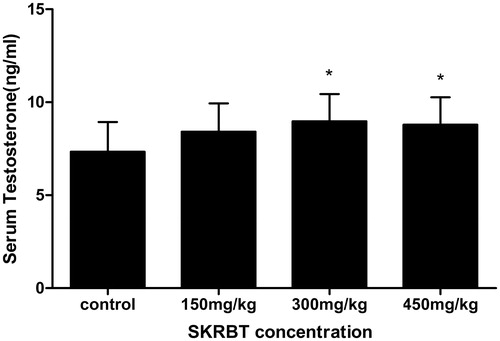
Changes in serum testosterone after the treatment of SKRBT
After treated with SKRBT, the average serum testosterone levels of all experimental groups seemed to be higher than the control group (). Among them, the effects of SKRBT at a dose of 300 and 450 mg/kg were significant.
Tests of male sexual behavior
Significant effects of SKRBT were observed in multiple measures of sexual behavior (). The administration of SKRBT shortened the mount latency at the dose of 300 mg/kg (p < 0.01). All three experimental groups had decreased intromission latency (p < 0.05). Although there was an upward trend of ejaculation latency after using SKRBT, no significant differences were seen between the control and experimental groups.
Figure 2. Measures of sexual behavior of control group and SKRBT treated groups (n = 12 per group). All values are mean ± SEM, #p < 0.01,*p < 0.05 compared with control group. (A) Mean latency in minutes of mount, intromission and ejaculation. (B) Mean frequency per minute of mount, intromission and mean copulatory efficacy per minute.
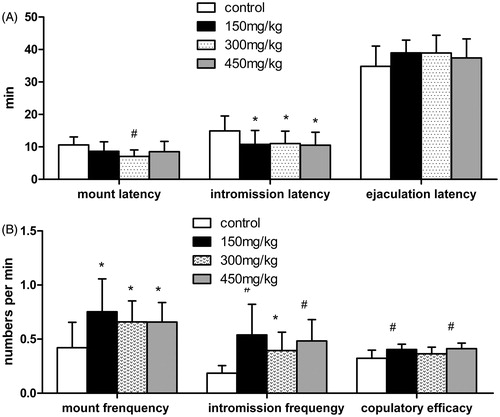
Comparison of the frequency of mount showed that all of the three experimental groups had a higher frequency of mount than the control group (p < 0.05). Similarly, the intromission frequency of the three experimental groups was also higher than the control group (p < 0.01, groups at the doses of 150 and 450 mg/kg; p < 0.05, group at the dose of 300 mg/kg). SKRBT significantly improved copulatory efficacy in groups treated with the dosage of 150 and 450 mg/kg (p < 0.01), and no statistical difference was observed in group at the dosage of 300 mg/kg.
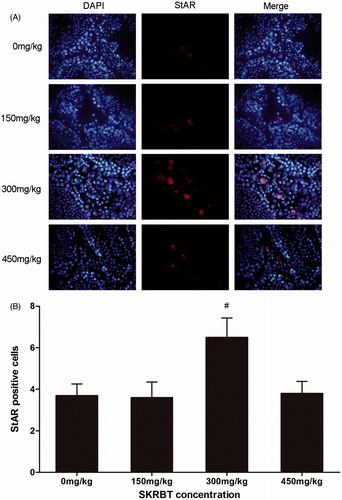
Immunofluorescence staining for StAR
According to the result of immunofluorescence staining, the number of StAR-positive Leydig cells in the group treated with SKRBT at the dosage of 300 mg/kg was markedly larger than that in control group (p < 0.05) ().
Figure 3. Mice were treated with SKRBT every day for three weeks and then the testes were harvested for fluorescent immunohistochemistry to identify the StAR-positive Leydig cells in interstitial spaces. (A) The number of StAR-positive cells (n = 12 per group) was determined. I = interstitial spaces, T = seminiferous tubules. Magnification ×400. (B) The number of StAR-positive cells per interstitial space. Results are shown as mean ± SEM (n = 12).
Discussion
It is well-documented that the serum testosterone decreases in men with aging by 1–2% per year after the age of 40 years [Citation23]. The European Male Ageing Study (EMAS) has defined the strict diagnostic criteria for LOH, including the presence of reproducibly low serum testosterone (total testosterone <11 nmol/land free testosterone <220 pmol/l) and three sexual symptoms (erectile dysfunction, lessened sexual thoughts and weakened morning erections) [Citation23]. Therefore, the presence of sexual symptoms is essential for the diagnosis of LOH. Data concerning the effect of testosterone on sexual function in men with LOH are limit so far. A recent metaanalysis article showed that TRT plays positive effects on male sexual function in hypogonadal patients [Citation25]. As mentioned earlier, although the potential adverse effects could not be ignored, TRT is still considered to be an effective therapy method for male hypogonadism.
Finding a new method to treat LOH and avoid the risks of TRT as much as possible is important. In China, the rate of Kampo medicine use was higher than that of testosterone because of the perfect performance and lower side effect.
Several articles reported that SKRBT could relieve the symptoms of LOH [Citation19,Citation20,Citation26]. To our knowledge, this is the first study to investigate the prospective effective effects of SKRBT in improving the sexual behaviors of aging mice. Twenty-four-month-old mouse was used as an LOH animal model for examining the effects of SKRBT on serum testosterone levels and sexual functions. According to the result, the levels of serum testosterone at the dosage of 300 and 450 mg/kg were improved by SKRBT. Although the groups of higher dose improved the serum testosterone levels, we still supposed that SKRBT might not work in a dose-dependent manner because the level of serum testosterone in the group of 450 mg/kg (8.80 ± 1.46) had a tendency to decline comparing with the group of 300 mg/kg (8.98 ± 1.47). A similar phenomenon was observed in castrated mice in a previous study [Citation26].
As noted earlier, TRT plays positive effects on male sexual function in hypogonadal patients [Citation25]. In order to investigate if the elevated levels of serum testosterone produced by SKRBT can improve the sexual behaviors of aging mice, we tested several relevant items. Our present results demonstrated that SKRBT can increase the number of mounts and intromissions before ejaculation but shorten the latency of them. The SKRBT-treated groups revealed a slightly longer latency of ejaculation in comparison to the controlled group, although no statistical significance was observed. Moreover, copulatory efficacy had been significantly improved by SKRBT. Mount latency and intromission latency are thought to be inversely proportional to sexual motivation, while ejaculation latency, intromission frequency and copulatory efficacy are considered to be indicative of potency [Citation21,Citation27]. That is, shorter latency of mount and intromission implies lower sexual desire, while longer ejaculate latency, higher frequency of intromission or copulatory efficacy mean increased potency. All of these results may suggest that SKRBT may have a favorable effect on sexual function in aging mice. During the treatment, no side effect was observed, meaning that SKRBT is a safe medicine.
The StAR protein mediates the rate-limiting and acutely regulated step in steroidogenesis, and plays an indispensable role in transferring cholesterol from the outer to the inner mitochondrial membrane where the cytochrome P450 side chain cleavage (P450scc) enzyme resides in and initiates the conversion of cholesterol to pregnenolone [Citation28]. The effects of signal molecules on the regulation of testicular functions have been studied earlier, but the influence of the herbal medicine on steroidogenesis has seldom been investigated. A previous study has documented that Gibberellic acid, a plant preparation, could increase the levels of StAR and enhance the activity of steroidogenic markers 3β-hydroxysteroid dehydrogenase (3β-HSD) and 17β-HSD in rats [Citation29]. Our results indicate that SKRBT can improve the serum level of testosterone through increasing the expression StAR. Michihara et al. elucidated that SKRBT could inhibit the activities of aromatase and then increase the serum testosterone levels in castrated mice [Citation26]. Tsujimura et al. revealed that the serum concentrations of testosterone fractions did not change after using SKRBT in 22 men over 40 years [Citation19]. This discrepancy occurred might due to a species difference between humans and mice or insufficient sample size. In addition, no serious adverse symptoms associated with SKRBT treatment were found. Therefore, treatment with SKRBT might be quite safe for elderly patients with LOH.
Our study suggests that SKRBT is a secure, effective way to increase the levels of serum testosterone and then improve the sexual functions of aging male mice. Up-regulation of rate-limiting enzyme of the testosterone synthesis process such as StAR might be the pharmacological basis of SKRBT on the treatment of LOH. But the sample size did not allow us to perform multivariate analysis for further investigation. At the current time, there still much to learn about the biological functions of SKRBT.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We thank Zhang Min, Xiao Wen Wang, Shi Zong Huang for their expert technical assistance in animal experiments.
References
- Mäkinen JI, Huhtaniemi I. Androgen replacement therapy in late-onset hypogonadism: current concepts and controversies - a mini-review. Gerontology 2011;57:193–202
- Araujo AB, Wittert GA. Endocrinology of the aging male. Best Pract Res Clin Endocrinol Metab 2011;25:303–19
- Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123–35
- Morales A, Lunenfeld B; International Society for the Study of the Aging Male. Investigation, treatment and monitoring of late-onset hypogonadism in males. Official recommendations of ISSAM. International Society for the Study of the Aging Male. Aging Male 2002;5:74–86
- Nieschlag E, Swerdloff R, Behre HM, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. ISA, ISSAM, and EAU recommendations. Eur Urol 2005;48:1–4
- Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. Int J Androl 2009;32:1–10
- Neaves WB, Johnson L, Porter JC, et al. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab 1984;59:756–63
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men:longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002;87:589–98
- Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab 2008;93:2737–45
- Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci 2002;57:M76–99
- Kenny AM, Bellantonio S, Gruman CA, et al. Effects of transdermal testosterone on cognitive function and health perception in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 2002;57:M321–5
- Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf) 2005;63:381–94
- Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 2010;95:639–50
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536–59
- McGill JJ, Shoskes DA, Sabanegh ES. Androgen deficiency in older men:indications, advantages, and pitfalls of testosterone replacement therapy. Cleve Clin J Med 2012;79:797–806
- Mizoguchi K, Yuzurihara M, Ishige A, et al. Saiko-karyukotsu-borei-to, a herbal medicine, ameliorates chronic stress-induceddepressive state in rotarod performance. Pharmacol Biochem Behav 2003;75:419–25
- Kanba S, Yamada K, Mizushima H, Asai M. Use of herbal medicine for treating psychiatric disorders in Japan. Psychiatry Clin Neurosci 1998;52:S331–3
- Sarai K. Oriental medicine as therapy for resistant depression: use of some herbal drugs in the far east (Japan). Prog Neuropsychopharmacol Biol Psychiatry 1992;16:171–80
- Tsujimura A, Takada S, Matsuoka Y, et al. Clinical trial of treatment with saikokaryukotsuboreito for eugonadal patients with late-inset hypogonadism-related symptoms. Aging Male 2008;11:95–9
- Tsujimura A, Miyagawa Y, Okuda H, et al. Change in cytokine levels after administration of saikokaryuukotsuboreito or testosterone in patients with symptoms of late-onset hypogonadism. Aging Male 2011;14:76–81
- Benelli A, Bertolini A, Zoli M, et al. Pharmacological manipulation of brain galaninergic system and sexual behavior in male mice. Psychopharmacology (Berlin) 2002;160:325–30
- Clemens LG, Wee BE, Weaver DR, et al. Retention of masculine sexual behavior following castration in male B6D2F1 mice. Physiol Behav 1988;42:69–76
- Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl 2014;16:192–202
- Taylor MF, Woolveridge I, Metcalfe AD, et al. Leydig cell apoptosis in the rat testes after administration of the cytotoxin ethane dimethanesulphonate:role of the Bcl-2 family members. J Endocrinol 1998;157:317–26
- Corona G, Isidori AM, Buvat J, et al. Testosterone supplementation and sexual function:a meta-analysis study. J Sex Med 2014;11:1577–92
- Michihara S, Shin N, Watanabe S, et al. A Kampoformula, saikokaryukotsuboreito, improves serum testosterone levels of castrated mice and its possible mechanism. Aging Male 2013;16:17–21
- Clark JT, Kalra SP, Kalra PS. Effects of a selective alpha 1-adrenoceptor agonist, methoxamine, on sexual behavior and penile reflexes. Physiol Behav 1987;40:747–53
- Walsh LP, McCormick C, Martin C, et al. Roundup inhibits steroidogenesis by disrupting steroidogenic acute regulatory (StAR) protein expression. Environ Health Perspect 2000;108:769–76
- Premalatha R, Jubendradass R, Srikumar K, et al. Gibberellic acid acts as an agonist of steroidogenesis in male rats. Andrologia 2013. [Epub ahead of print]