Abstract
Objectives: Around 40% of diabetic men have lowered testosterone and symptoms of hypogonadism but the prevalence of hypogonadism among prediabetic men is unknown. The aim of this study was to investigate the prevalence of late-onset hypogonadism (LOH) in population of Polish men with prediabetes.
Methods: This study was performed in 196 prediabetic men and in 184 normoglycemic, control group. Prediabetes was defined as impaired fasting glucose, impaired glucose tolerance and/or HbA1c 5.7–6.4%. LOH was defined as low libido, diminished frequency of morning erections and erectile dysfunctions in men with total testosterone <12 nmol/l.
Results: Total testosterone (TT) level in prediabetes group was 11.78 ± 1.76 and 16.37 ± 1.6 nmol/l in control group (p < 0.001). LOH was diagnosed in 30% prediabetic men and in 13.6% control men. There were negative relationships between calculated free testosterone (cFT) and HbA1c (r = −0.3856; p < 0.005). In prediabetic group, TT and cFT levels were lower in patients with impaired glucose tolerance than impaired fasting glucose (p < 0.05 and p < 0.02, respectively). We showed inverse relationships between IIEF-5 score and cFT (r = −0.414, p < 0.005) and between IIEF-5 and HbA1c (r = −0.395, p < 0.002).
Conclusions: In population of Polish men with prediabetes we observed high prevalence of LOH. Routine testosterone screening should be performed in all prediabetic men.
Introduction
Aging population, obesity and hormonal deficiencies have resulted in the great rise in the prevalence of metabolic disorders in men. Prediabetes is a condition in which the patients have slight increase in glucose concentrations but they are not said to be diabetic. The American Diabetes Association (ADA) defines prediabetes as impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and/or glycated hemoglobin (HbA1c) of 5.7–6.4% [Citation1]. Prediabetes patients are likely to develop diabetes mellitus type 2 (T2DM) and they are also in high risk of cardiovascular complications [Citation2]. The prevalence of prediabetes in Poland is one of the highest in the world – about 16.5% of Polish population, compared with 7% of the world population, is estimated to have IGT and by the year 2035, the number of patients is projected to reach to 19.3% [Citation3].
Low testosterone levels in T2DM men were described in 2004 [Citation4] and are present in about 30–40% of patients [Citation5,Citation6]. Hypogonadism in diabetic men is associated with reduced insulin sensitivity [Citation7], so a causal role for low testosterone in the development of T2DM is suggested in men [Citation8,Citation9]. The relationship between testosterone and T2DM is an important issue, given the fact that T2DM is becoming a fast-growing epidemic, the morbidity associated with which is more disabling than the disease itself. Various studies have demonstrated the increasing prevalence of hypogonadism in diabetic subjects, but whether this is a cause or effect is still an area of active research [Citation10]. Also prediabetes is characterized by insulin resistance and hypogonadism is an independent risk factor of high fasting plasma glucose (FPG) leading to an increased prevalence of IGT [Citation11]. Male aging is associated with a decline in total testosterone (TT) levels approximately 1–2% per year [Citation12] leading to an increase of the prevalence of late-onset hypogonadism (LOH) in men [Citation13].
Only few studies have demonstrated relationships between androgens and prediabetes in men [Citation14,Citation15]. In our previous study we also showed [Citation16] the high prevalence of prediabetes among patients with LOH. These studies were performed in patients with erectile dysfunctions (ED), LOH or with IFG but not in patients with prediabetes. The influence of prediabetes on testosterone deficiency is still unknown, thus the aim of this study was to investigate the prevalence of LOH among old and middle-aged males with prediabetes in Polish population and possible relationships with comorbidities.
Materials and methods
This study was performed in Department of Internal Diseases, Diabetology and Endocrinology, Medical University of Warsaw, Poland. We recruited 196 prediabetic men, aged 50–75 years and 184 normoglycemic control men. All protocols and procedures were approved by Medical University of Warsaw Research Ethics Committee and where appropriate. All patients gave written informed consent.
We excluded patients with T2DM, insufficiency of hypophysis, primary hypogonadism, hyperprolactinemia and those who received testosterone replacement therapy or androgen deprivation therapy. Demographic parameters, clinical history including comorbidities, medications and the presence of erectile dysfunction (ED) and cardiovascular disease (CVD) were collected. Fasting plasma glucose (FPG), oral glucose tolerance test (OGTT) and HbA1c were measured.
Prediabetes was diagnosed in patients with IFG from 100 to 125 mg/dl and two-hours glucose concentration in OGTT <140 mg/dl or in patients with IGT – two-hour glucose concentration in OGTT from 140 to 200 mg/dl or in patients with HbA1c from 5.7% to 6.4% [Citation1]. The diagnosis of metabolic syndrome (MetS) was based on the following criteria: waist circumference ≥94 cm and any two of the following: triglycerides ≥150 mg/dl, HDL-cholesterol <40 mg/dl, blood pressure ≥130/85 mmHg and FPG ≥100 mg/dl [Citation17].
LOH was diagnosed in patients with symptoms of testosterone deficiency like: low libido, diminished frequency of morning erections and ED. One or more of these symptoms must be corroborated with a decreased TT levels <12 nmol/l or free testosterone levels <0.255 nmol/l [Citation13]. Erectile function was assessed according to the International Index of Erectile Function (IIEF-5) questionnaire [Citation18]. Possible scores on the IIEF-5 are 1–25 and ED was classified into five categories based on the scores: severe – 1–7, moderate – 8–11, mild to moderate – 12–16, mild – 17–21 and none – 22–25.
Height, weight and waist circumference were measured and body mass index (BMI) was calculated. Obesity was defined as BMI (the weight in kilograms divided by the square of the height in meters) of 30 or more. CVD was defined as self-reported coronary artery disease, a history of myocardial infarction or stroke, congestive heart failure or arrhythmia. Hypertension was considered to be present if the participant reported having received the diagnosis or if he was receiving medication for the condition.
Fasting blood samples were then obtained between 8:00 and 10:00 A.M. to measure serum TT, estradiol (E2), sex hormone binding globuline (SHBG), luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin, FPG and HbA1c. All men had their hormones levels checked at least once. TT, LH, FSH, SHBG, DHEA and PRL were measured by chemiluminescent immunometric and radioimmunoassay methods (Immulite 2000 and RIA CAC; Siemens Medical Solution, Malvern, PA, USA). The normal value for testosterone was 12–28 nmol/l, for LH: 2–6 mIU/l, for FSH: 3–10 mIU/l, for PRL: 12–24 ng/ml and for DHEA-S: 200–375 ng/dl. Calculated free testosterone (cFT) was calculated from SHBG, serum albumin and TT using the method of Vermeulen [Citation19] and cFT level <0.255 nmol/l was taken as low.
Statistical analysis was performed using Statistica 10 software (StatSoft, Tulsa, OK, USA). To establish correlation, Sperman test was used to compare nonparametric data and Pearson test to compare parametric data. All relationships were assessed by linear univariate and multivariate regression analysis to reduce bias in a cross-sectional study. In multivariate analysis, statistical data were adjusted for age, BMI and MetS. Results were considered statistically significant at p < 0.05.
Results
A total of 196 men, mean age 68.4 ± 3.1 years with prediabetes and 184 normoglycemic control men, mean age 65.8 ± 3.4 years were evaluated. Characteristic of both groups is shown in . The mean TT and cFt levels in prediabetes group were lower than observed in control men (p < 0.001). We observed higher E2 levels in prediabetes group (p < 0.002); LH, FSH and SHBG levels were also higher in prediabetes group (p < 0.05) but DHEA and prolactin levels did not differ significantly. BMI and waist circumference were higher in prediabetes group (p < 0.01 and p < 0.02, respectively) as well as HbA1c (p < 0.02). In prediabetic group we observed significantly higher levels of total cholesterol, LDL-cholesterol and triglicerydes, while HDL-cholesterol levels were lower – .
Table 1. Characteristic of patients with prediabetes and control group.
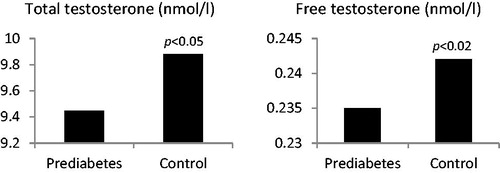
LOH was diagnosed in 60 (30%) prediabetic men and in 25 (13.6%) control men. In LOH subgroup of prediabetic patients mean TT level was 9.45 ± 0.8 nmol/l compared with 14.34 ± 1.1 nmol/l in eugonadal prediabetic men (p < 0.002). In control group with LOH mean TT level was 9.88 ± 0.9 nmo/l compared with 15.25 ± 1.3 nmol/l in eugonadal control men (p < 0.002). The mean TT levels in prediabetic group with LOH were lower than in control men with LOH (p < 0.05) – . We also observed significant differences of cFT levels between groups. In prediabetic men with LOH mean cFT level was 0.235 ± 0.08 nmol/l compared with 0.356 ± 0.09 nmol/l in eugonadal prediabetic men (p < 0.001). In control group with LOH mean cFT level was 0.242 ± 0.9 nmo/l compared with 0.388 ± 0.08 nmol/l in eugonadal control men (p < 0.001). The mean cFT levels in prediabetic group with LOH were lower than observed in control group with LOH (p < 0.02) – .
Figure 1. Total and calculated free testosterone concentrations in men with LOH in prediabetes and control group.
In prediabetic group IFG was diagnosed in 102 men (52%) and IGT in 94 (48%). In 188 (96%) prediabetic men, HbA1c was found in range from 5.7% to 6.4%. From 60 prediabetic man with LOH, IFG was diagnosed in 32 (53%) and IGT in 28 (47%). HbA1c in range from 5.7 to 6.4 was observed in all patients with LOH.
Analysis of prediabetic group with LOH revealed, that patients with IGT had lower TT and cFT levels than all prediabetic group and subgroup with IFG (p < 0.05 and p < 0.02; respectively) – . In multivariate analysis these relationships were significant after adjustment for age, BMI and MetS.
Table 2. Total testosterone (TT) and calculated free testosterone (cFT) concentrations in prediabetic men with LOH after dividing according glycemic control disorders.
In prediabetes group we have shown negative relationships between BMI and TT (r = −0.3641; p < 0.01) and between BMI and cFT (r = −0.3724, p < 0.01) as well as between waist circumference and TT or cFT (r = −0.3452; p < 0.05 and r = −0.3646; p < 0.02, respectively). There were also negative relationships between cFT and HbA1c (r = −0.3856; p < 0.005) and between cFT and LDL-cholesterol (r = −0.3576, p < 0.02) but TT levels correlated significantly only with LDL-cholesterol (r = −0.3546, p < 0.02). In multivariate analysis these relationships were significant after adjustment for age, and BMI.
In patients with prediabetes 27% had mild, 27% had mild to moderate, 21% moderate and 25% severe ED. Linear regression analysis showed inverse relationships between IIEF-5 score and TT (r = −0.354, p < 0.02) and between IIEF-5 score and cFT (r = −0.414, p < 0.005). These relationships were significant after adjustment for HbA1c, age, BMI and MetS. We also observed inverse relationship between IIEF-5 and HbA1c (r = −0.395, p < 0.002). This relationship was significant after adjustment for age, BMI and MetS.
The prevalence of comorbidities in both groups is shown in . In prediabetic group obesity was diagnosed in above 80%, MetS in 66% and differences with control group were significant (p < 0.02). The prevalence of CVD was also significantly higher in prediabetic group (p < 0.05) but prevalence of hypertension and smoking did not differ between groups. We have also shown significant differences of the prevalence of obesity and MetS between patients with LOH and eugonadal men among prediabetic group (p < 0.02 and p < 0.05, respectively) – .
Table 3. The prevalence (no. pts., %) of comorbidities in prediabetic men and in control group.
Discussion
Disorders of glucose metabolism are an established risk factor for low testosterone levels and ED, but the prevalence of hypogonadism in prediabetic patients is still unknown. In our study we investigated the prevalence of LOH among old and middle-aged males with prediabetes in Polish population and relationships with comorbidities. This study was performed in the relative large population of Eastern European men and our results showed high prevalence of LOH in prediabetic men with multiple comorbidities.
We have demonstrated that in prediabetic men LOH was diagnosed in 30% of patients and only in 13.6% in control group. We also observed lower TT and cFT levels in prediabetic men compared with control group as well as lower TT and cFT levels in prediabetic patients with LOH compared with control group with LOH. These relationships were significant after adjustment for age, BMI and MetS. In prediabetic patients we also showed negative relationships between androgens levels and HbA1c as well as LDL-cholesterol. These relationships also were significant after adjustment for age and BMI.
In our study prediabetes was diagnosed in patients with IFG, IGT and/or HbA1c from 5.7–6.4% [Citation13]. Currently, an intermediate HbA1c range is not considered as prediabetes by the World Health Organization, however, this identification of prediabetes probably gives wider range of subjects at the risk group of T2DM development [Citation20]. We also observed that patients with IGT had lower TT and cFT levels than all prediabetic group and subgroup with IFG and these relationships were significant after adjustment for obesity and MetS. These results indicate that low testosterone is independently associated with prediabetes and probably may affect rather on glucose tolerance than on fasting glucose concentration. Regardless of these details associated with diagnosis, LOH in patients with prediabetes in Polish population was very common and should be taken under consideration during evaluation of patients with prediabetes because some studies, discussed below, have suggested beneficial effects of testosterone therapy on glucose metabolism.
The prevalence of prediabetes among men in Poland is one of the highest in the world and this may be associated with high prevalence of T2DM and high prevalence and incidence of LOH. In this study the prevalence of LOH in prediabetic patients was more than twice compared with normoglycemic men while in our previous study we also observed the high prevalence of prediabetes among patients with LOH [Citation16]. We observed prediabetes in about 40% hypogonadal patients and only in 13% in control group. Mean TT and cFT levels were lower in prediabetic patients and correlated negatively with HbA1c. These results are similar to the current findings and indicate that in Polish population low testosterone levels and prediabetes are closely related to each other.
ED is considered as the most prominent symptomatic reflection of LOH [Citation13]. Similarly, T2DM in men is associated with lower testosterone levels and the majority of these men have signs and symptoms of hypogonadism such as ED, low libido, fatigue, sarcopenia and depression [Citation3] but hypogonadism in diabetic men might have been partially explained by several modifiable risk factors like MetS, obesity and dyslipidmia [Citation21,Citation22].
We showed previously the high prevalence of hypogonadism among patients with ED [Citation23]. In this study about 50% patients with ED had TT levels <12 nmol/l and there was inverse correlation between IIEF-5 score and TT levels. In the current study, we also observed relationship between TT levels and IIEF-5 score in patients with prediabetes. In another study we showed that 46% of T2DM men was hypogonadal and there was inverse relationship between HbA1c and TT levels [Citation24]. This relationship indicates that low TT levels were connected with poor glycemic control. In the current study we also documented negative relationships between androgens and HbA1c. In our opinion these observations confirm significant relationships between androgens and T2DM and also prediabetes in men.
Only few studies have demonstrated relationships between androgens and prediabetes in men. In MEST study [Citation25] negative relationship between testosterone and IFG was observed. Also association of prediabetes with an increased risk of testosterone deficiency was demonstrated [Citation14,Citation15]. These cross-sectional studies were performed in populations of relatively healthy men, when compared with Polish population with multiple comorbidities.
The possible pathophysiological mechanism between prediabetes and low testosterone is probably multifactoral and represented mainly by visceral adiposity and insulin resistance. Low testosterone levels in men are associated with insulin resistance and reduced insulin sensitivity [Citation7] and have been found to predict insulin resistance, obesity and T2DM [Citation26]. Testosterone can be converted to estradiol in the adipose tissue and it has been suggested that excessive estrogens synthesis in obese men may suppress secretion of testosterone [Citation27]. In our study estradiol levels were higher in men with LOH and prediabetes. The studies in animals and men after androgen deprivation therapy which suppress functions of androgen/androgen receptor (AR) signaling revealed significant metabolic disorders like T2DM and MS which can be associated with tissue-specific AR signaling that is involved in regulation of metabolism [Citation28]. Also AR positive cells number was evaluated in 259 men with T2D [Citation29] and authors demonstrated that AR was negatively correlated with the course of T2DM and that low levels of TT and AR may be potential risk factors for T2DM in elderly men. Recent studies have also shown that nucleotide polymorphism of the endothelial nitric oxide synthase (eNOS) gene may be an independent risk factor for T2DM and insulin resistance in men with LOH [Citation30].
Low TT is associated not only with increased risk of incident T2DM in men [Citation31] but also cardiovascular events and death, therefore possible testosterone deficiency should be diagnosed in men with prediabetes [Citation12]. Evidence derived from clinical studies supports the use of testosterone replacement in hypogonadal patients, although the benefit–risk ratio is uncertain in advanced age. Testosterone replacement therapy in diabetic men may improve insulin sensitivity, glycemic control, cardiovascular risk factors and diabetic complications [Citation32–36] but long-term influence of testosterone in men with LOH and prediabetes is still unknown.
Some of the issues can be cited as weaknesses in our data set. The testosterone measurements were not repeated in our sample set. The most widely accepted parameter to establish the presence of hypogonadism is the measurement of TT. Unfortunately, no consensus has been reached regarding the lower TT threshold defining LOH and there are no generally accepted lower limits of normal TT [Citation13]. In our study we have chosen 12 nmol/l as a cutoff for low testosterone low limits of normal range because the prevalence of LOH symptoms increases with TT levels <12 nmol/l [Citation37]. It is also important to remember that our model in no way established a causal link between testosterone and prediabetes; these conditions might simply overlap and they have probably separate pathophysiologic pathways.
Conclusions
In population of Polish men with prediabetes we observed high prevalence of LOH and routine testosterone measurements should be performed in all men with glucose metabolism disorders.
Declaration of interest
The authors declare that they have no conflict of interest.
This study was supported by research grant number 501-2-1-07-21/09 of the Medical Centre for Postgraduate Education, Poland.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37:S81–90
- Rydén L, Grant PJ, Anker SD, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2014;27:133–73
- Cho NH, Whiting D, Guariguata L. IDF diabetes atlas. 6th ed. Brussels, Belgium: International Diabetes Federation; 2013
- Dhindsa S, Prabhakar S, Sethi M, et al. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004;89:5462–8
- Corona G, Mannucci E, Petrone L, et al. Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int J Impot Res 2006;18:190–7
- Kapoor D, Aldred H, Clark S, et al. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007;30:911–7
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008;93:1834−40
- Stellato RK, Feldman HA, Hamdy O, et al. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts Male Aging Study. Diabetes Care 2000;23:490–4
- Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004;27:1036–41
- Beatrice AM, Dutta D, Kumar M, et al. Testosterone levels and type 2 diabetes in men: current knowledge and clinical implications. 1. Diabetes Metab Syndr Obes 2014;7:481–6
- Mazur A, Westerman R, Werdecker A, Mueller U. Testosterone and type 2 diabetes in men. Aging Male 2014;1:18–22
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2002;87:589−98
- Lunenfeld B, Mskhalaya G, Kalinchenko S, Tischova T. Recommendations on the diagnosis, treatment and monitoring of late-onset hypogonadism in men – a suggested update. Aging Male 2013;4:143–50
- Corona G, Rastrelli G, Balercia G, et al. Hormonal association and sexual dysfunction in patients with impaired fasting glucose: a cross-sectional and longitudinal study. Sex Med 2012;9:1669–80
- Ho CH, Yu HJ, Wang CY, et al. Prediabetes is associated with an increased risk of testosterone deficiency, independent of obesity and metabolic syndrome. PLoS One 2013;8:e74173. doi:10.1371/journal.pone.0074173
- Rabijewski M, Papierska L, Piątkiewicz P. The prevalence of prediabetes in population of Polish men with late-onset hypogonadism. Aging Male 2014;17:141–146
- Athyros GV, Ganotakis ES, Mikhailidis DO. The prevalence of the metabolic syndrome using the National Cholesterol Educational Program and International Diabetes Federation definitions. Curr Med Res Opin 2005;21:1157–64
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319–26
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72
- Saukkonen T, Cederberg H, Jokelainen J, et al. Limited overlap between intermediate hyperglycemia as defined by A1C. 5.7–6.4%, impaired fasting glucose, and impaired glucose tolerance. Diabetes Care 2011;34:2314–16
- Kim ML, Rolland O, Cepeda JK, et al. Diabetes mellitus in older men. Aging Male 2006;9:139–47
- Atlantis E, Lange K, Martin S, et al. Testosterone and modifiable risk factors associated with diabetes in men. Maturitas 2011;68:279–85
- Rabijewski M, Papierska L, Kozakowski J, Zgliczyński W. The high prevalence of testosterone deficiency in population of Polish men over 65 years with erectile dysfunctions. Aging Male 2012;15:258–62
- Rabijewski M, Papierska L, Zgliczyński W, Piątkiewicz P. The Incidence of hypogonadotropic hypogonadism in type 2 diabetic men in Polish population. Biomed Res Int 2013;2013:767496. doi:10.1155/2013/767496
- Colangelo LA, Ouyang P, Liu K, et al. Association of endogenous sex hormones with diabetes and impaired fasting glucose in men: Multi-Ethnic Study of Atherosclerosis. Diabetes Care 2009;32:1049–51
- Salving E, Finley M, Zhang L, et al. Androgens and diabetes in men. Results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care 2007;30:234–8
- Pitteloud N, Dwyer AA, DeCruz S, et al. The relative role of gonad sex steroids and gonadotropin-releasing hormone pulse frequency in the regulation of follicle-stimulating hormone secretion in men. J Clin Endocrinol Metab 2008;93:2686–92
- Yu IC, Lin HY, Sparks JD, et al. Androgen receptor roles in insulin resistance and obesity in males: the linkage of androgen-deprivation therapy to metabolic syndrome. Diabetes 2014;63:3180–8
- Cao J, Li J, Hao W, et al. Correlation of sex hormone and androgen receptor with diabetes mellitus in elderly men. Aging Male 2011;14:162–7
- Delli Muti N, Tirabassi G, Lamonica GR, et al. Diabetes mellitus and late-onset hypogonadism: the role of Glu298Asp endothelial nitric oxide synthase polymorphism. Andrologia 2014. [Epub ahead of print] doi:10.1111/and.12339
- Schipf S, Haring R, Friedrich N, et al. Low total testosterone is associated with increased risk of incident type 2 diabetes mellitus in men: results from the Study of Health in Pomerania (SHIP). Aging Male 2011;14:168–75
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010;7:3495–03
- Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 2011;34:828–37
- Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with “diabesity": results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol 2014. 2014:683515. doi:10.1155/2014/683515
- Haider A, Saad F, Doros G, Gooren L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational study. Obes Res Clin Pract 2014;8:e339–49
- Janjgava S, Zerekidze T, Uchava L, et al. Influence of testosterone replacement therapy on metabolic disorders in male patients with type 2 diabetes mellitus and androgen deficiency. Eur J Med Res 2014;19:56
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab 2006;91:4335–43
