Abstract
Background: There has been a longstanding question as to whether testosterone therapy could precipitate or worsen urinary symptoms in aging men. We investigated the effects of 1-year oral testosterone undecanoate (TU) therapy on urinary symptoms in aging, hypogonadal men.
Methods: A total of 322 men ≥50 years with symptomatic testosterone deficiency participated in a 1-year, randomized, multicenter, double-blind trial. Patients received placebo or oral TU 80 mg/day, 160 mg/day, or 240 mg/day.
Results and limitations: Compared with placebo, treatment with oral TU at doses of 80 mg/day and 160 mg/day resulted in no significant change in IPSS urinary symptoms or quality of life (QoL) scores. Treatment with oral TU 240 mg/day led to a statistically significant, but clinically insignificant, improvement in IPSS total score and a significant improvement in IPSS QoL score. None of the TU doses tested had a significant effect on PSA or PV.
Conclusions: Long-term oral TU therapy had no deleterious effects on IPSS total score and did not change PV and PSA in aging, hypogonadal men. Oral TU therapy at a dose of 240 mg/day may even improve IPSS QoL score.
Introduction
Andriol® Testocaps® is an oral testosterone undecanoate (TU) formulation that is taken with normal meals. Following TU’s intestinal absorption with ingested meal lipids, it is incorporated into post-meal chylomicrons and transported to the systemic circulation by the intestinal lymphatic system, thereby circumventing the portal circulation and liver and avoiding both rapid metabolism and the potential hepatotoxicity that has been reported with other oral TRTs [Citation1,Citation2]. Following its release from circulating post-meal chylomicrons, TU is rapidly hydrolyzed by esterases, liberating testosterone [Citation2].
We conducted a 1-year, randomized, placebo-controlled trial investigating the effects of three daily doses of oral TU (80 mg/day, 160 mg/day, and 240 mg/day; taken as divided doses with normal meals) in 322 men at least 50 years of age with symptomatic hypogonadism based on a positive score on the Androgen Deficiency in Aging Males (ADAM questionnaire) [Citation3] and a calculated morning free testosterone measurement of <0.26 nmol/L [Citation4,Citation5]. This trial included a careful examination of the effects of oral TU on urinary symptoms as well as prostate-specific antigen (PSA) and prostate volume over the 1-year study period. The present report presents the findings of these assessments.
Methods and materials
This was a multicenter, randomized, double-blind, placebo-controlled trial, involving 14 centers in seven European countries. The trial was conducted between November 2001 (first subject entered) and July 2004 (last assessment of the last subject). This trial was registered at www.clinicaltrials.gov under number NCT00434824.
Study population
To determine eligibility for inclusion in the study, blood samples were collected in the morning (between 6 and 10 am) to measure levels of total testosterone and sex hormone-binding globulin (SHBG) for calculation of free testosterone using the formula by Vermeulen et al. [Citation6]. Men were eligible for inclusion in the study if they were at least 50 years of age, had a body mass index (BMI) between 18 and 34 kg/m2, and had symptomatic hypogonadism (as identified by a positive ADAM questionnaire score [Citation3] and a morning-calculated free testosterone level of <0.26 nmol/L) [Citation6]. Detailed exclusion criteria for the present study have been published previously [Citation5]. Patients whose cause of androgen deficiency was other than aging were excluded from the study. The study was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards and local regulatory agencies. All men gave written informed consent.
Intervention
Patients were treated for 12 months with six capsules per day, each containing 40 mg TU (Andriol Testocaps) or placebo, in divided doses with breakfast, lunch, and dinner. Eligible patients were randomized to one of four treatment groups: placebo, oral TU 80 mg/day (80 mg once daily), oral TU 160 mg/day (80 mg twice daily), or oral TU 240 mg/day (80 mg 3 times daily).
IPSS
At screening and Months 6 and 12, patients were to complete the International Prostate Symptom Score (IPSS) questionnaire, a symptom index for benign prostatic hyperplasia [Citation4]. The IPSS questionnaire involved eight questions: seven urinary symptoms questions and one global quality of life (QoL) question. The seven symptoms questions evaluated voiding (feeling of incomplete bladder emptying, intermittency, weak stream, and straining) and storage (frequency, urgency, and nocturia) symptoms. Each of the symptoms questions related to what the patient experienced during the previous month and involved assignment of a score from 0 to 5 for a maximum total of 35 points. The 8th global QoL question was assigned a score of 0 to 6. Lower IPSS values indicate better symptoms/QoL.
PSA
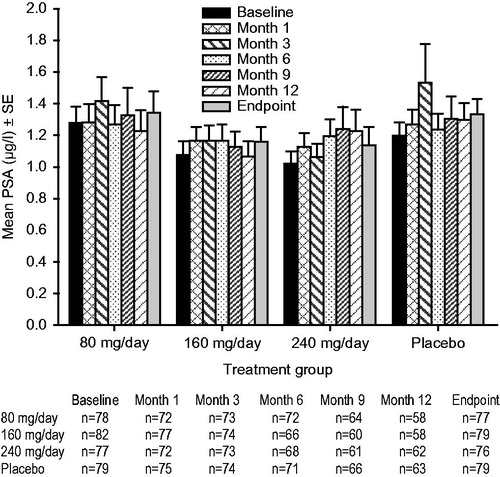
At screening, baseline, and Months 1, 3, 6, 9, and 12, blood samples were obtained for measurement of serum PSA level.
Prostate volume
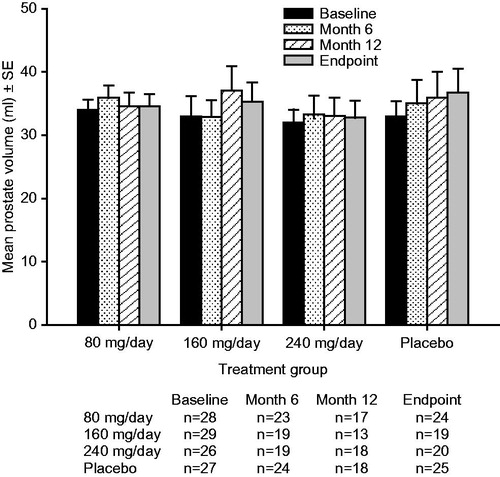
At sites where transrectal ultrasonography (TRUS) was available and patients consented, prostate volume was to be measured using TRUS at screening and months 6 and 12 (or at discontinuation). Since prostate examination by TRUS could influence blood levels of PSA, this measurement was performed after blood sampling for PSA.
Statistical analysis
The total score for the IPSS (IPSS-T) was calculated as the sum of the scores for the 7 symptoms questions. Scores for the voiding (IPSS-V) and storage (IPSS-S) symptoms subdomains were calculated as the sums of the scores for the IPSS questions relating to voiding and storage symptoms, respectively. The statistical significance of between-group differences was determined using analysis of variance (ANOVA). All ANOVA models included terms for treatment and region. The correlation between change from baseline at study endpoint in the IPSS total score and prostate volume was determined using the Pearson product–moment correlation, with the p-value for the correlation being determined using Fisher’s z-transformation under the null hypothesis that the correlation = 0.
Results
Patient demographics
A total of 1444 men were screened for eligibility; of these, 322 patients met the study inclusion/exclusion criteria and were randomized to receive oral TU (80 mg/day, 160 mg/day, or 240 mg/day) or placebo for up to 12 months. At screening, patients had a mean ± SD calculated free testosterone of 0.21 ± 0.04 nmol/l. The baseline clinical characteristics of the entire randomized population are shown in . At baseline, patients had a mean ± SD age of 58.7 ± 5.8 yr and a mean ± SD BMI of 27.3 ± 3.4 kg/m2. The overall mean ± SD prostate volume was 33.0 ± 12.6 ml, the mean ± SD PSA level was 1.15 ± 0.78 µg/l, and the mean ± SD IPSS total score (IPSS-T) was 5.6 ± 3.8 prior to treatment. The study population consisted of hypogonadal (testosterone <0.22 nmol/l) males who were generally overweight or obese (BMI >27 kg/m2), with PSA levels <4.0 µg/l, slightly enlarged prostates (>30 and <40 ml), and lower urinary tract symptoms of mild severity (IPSS-T score >0 and ≤7). The TU 160 mg/day group had slightly lower mean baseline IPSS-T score and IPSS subdomain scores (i.e. IPSS-V, IPSS-S, and QoL) compared with the other treatment groups. However, there were no clinically meaningful differences among the treatment groups with respect to baseline characteristics, including IPSS-T, IPSS-V, IPSS-S, and QoL scores.
Table 1. Baseline characteristics.
IPSS
The mean IPSS-T (), QoL (), IPSS-S (), and IPSS-V () scores remained generally stable over time for the TU 80 mg/day, TU 160 mg/day, and placebo groups. The only exception was that the placebo group showed small, progressive increases from baseline (i.e. worsening of symptoms) in the mean IPSS-T and IPSS-V scores. Relative to placebo, there were no significant between-group changes from baseline in the mean IPSS-T, QoL, IPSS-S, or IPSS-V scores following treatment with TU 80 mg/day or 160 mg/day ().
Figure 1. Mean ± standard error (SE) total International Prostate Symptom Score (IPSS) over time for testosterone undecanoate 80–240 mg/day versus placebo.
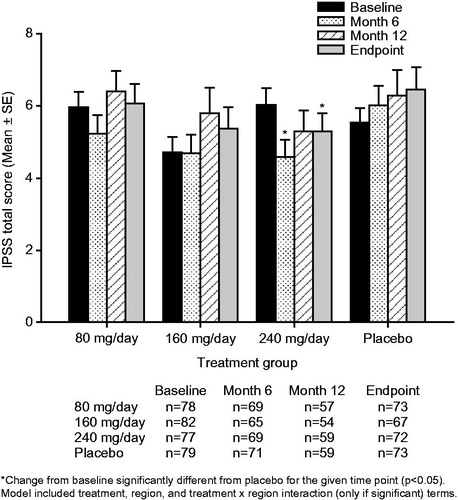
Figure 2. Mean ± standard error (SE) International Prostate Symptom Score (IPSS) quality of life subscore over time for testosterone undecanoate 80–240 mg/day versus placebo. Decrease in score = improvement.
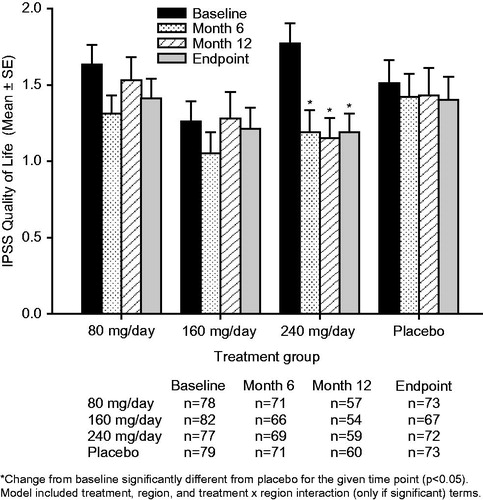
Figure 3. Mean ± standard error (SE) International Prostate Symptom Score (IPSS) storage subscore over time for testosterone undecanoate 80–240 mg/day versus placebo.
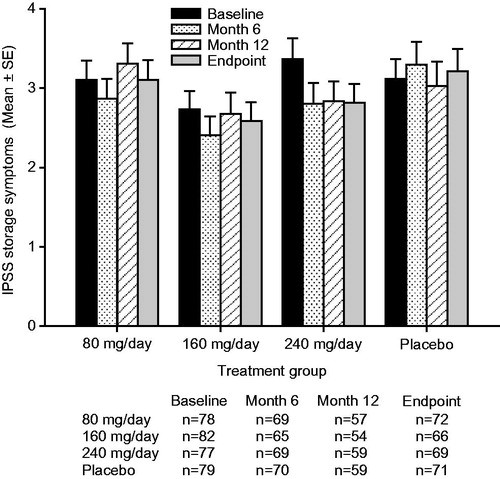
Figure 4. Mean ± standard error (SE) International Prostate Symptom Score (IPSS) voiding subscore over time for testosterone undecanoate 80–240 mg/day versus placebo.
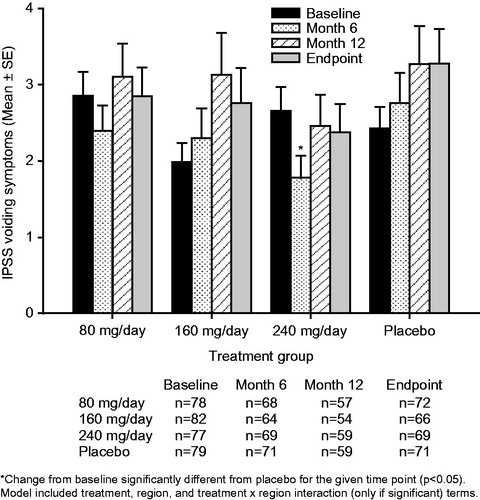
Table 2. Placebo-adjusted change from baseline in prostate-related measurements following treatment with testosterone undecanoate for up to 12 months (all subjects treated).
Treatment with TU 240 mg/day resulted in mean (95% confidence interval [CI]) placebo-adjusted reductions in IPSS-T scores of −1.3 [−2.5, −0.2] at Month 6, −0.9 [−2.3, 0.6] at Month 12 and −1.3 [−2.6, −0.0] points at study endpoint (). The improvements from baseline in the IPSS-T score seen with TU 240 mg/day reached significance relative to placebo both at Month 6 (p = 0.022) and study endpoint (p = 0.042). Treatment with TU 240 mg/day led to sustained mean [95% CI] placebo-adjusted reductions (improvements) in QoL scores of −0.4 [−0.8, −0.1] at Month 6, −0.5 [−0.9, −0.1] at Month 12 and −0.5 [−0.8, −0.1] at study endpoint (). The improvements from baseline in the QoL score with TU 240 mg/day were significant relative to placebo at Month 6 (p = 0.026), Month 12 (p = 0.018) and study endpoint (p = 0.014). A significant (p = 0.016) placebo-adjusted reduction from baseline in the IPSS-V score of −1.0 [95% CI: −1.8, −0.2] was seen in the TU 240 mg/day group at Month 6 (). The initial improvement in the IPSS-V score seen following 6-months of treatment with TU 240 mg/day was not sustained over the course of the trial, as significant placebo-adjusted differences in the IPSS-V score were not observed at either Month 12 or study endpoint. Patients treated with TU 240 mg/day also showed a trend toward small, sustained reductions from baseline in the IPSS-S score over time (); however, the placebo-adjusted differences in the IPSS-S score seen with TU 240 mg/day therapy did not reach significance at any of the time points examined ().
The number (%) of patients who had a clinically significant improvement [≥4-point decrease] [Citation7], no clinically significant change [change of 0–4 points] [Citation7], or clinically significant deterioration [≥4-point increase] [Citation7] from baseline in IPSS-T and their mean change from baseline in IPSS-QoL at study endpoint is summarized in . There appeared to be a small numerical improvement relative to placebo in the number and percentage of patients with clinically significant changes in IPSS-T score in the 240 mg/day group (higher percentage of patients had a clinically significant improvement and lower percentage had a clinically significant deterioration compared with placebo).
Table 3. Number (%) and mean change from baseline in IPSS quality of life score of patients who had a clinically significant improvement, no clinically significant change, or clinically significant deterioration from baseline in IPSS total score at study endpoint.
PSA and prostate volume
The mean PSA values at baseline, Months 1, 3, 6, 9, 12 and study endpoint, and mean prostate volume values at baseline, Month 6, Month 12 and study endpoint for each treatment group are shown in and , respectively. Within each treatment group, the values for mean PSA and mean prostate volume remained generally stable over the duration of the trial. There were no significant between-group differences in change from baseline for PSA or prostate volume with oral TU 80 mg/day, 160 mg/day, or 240 mg/day compared with placebo at any time point in this trial. The 95% CIs for the placebo-adjusted differences in change from baseline for PSA and prostate volume included zero at every time point for all TU doses ().
Correlation between prostate volume and IPSS-T score
The magnitudes of the changes from baseline in the IPSS-T score and the magnitudes of the changes from baseline in prostate volume were not significantly correlated at study endpoint for any of the treatment groups: TU 80 mg/day (r = −0.19; p = 0.383), 160 mg/day (r = 0.34; p = 0.160), 240 mg/day (r = −0.27; p = 0.253), and placebo (r = −0.05; p = 0.829).
Discussion
One of the key concerns with long-term use of TRT in aging, hypogonadal men is its effects on urinary symptoms [Citation8]. Our study is the largest long-term (1-year), placebo-controlled, dose-ranging trial conducted to date examining the effects of oral TU therapy on urinary symptoms, as assessed using the IPSS questionnaire, in aging (≥50 years) men with symptomatic hypogonadism. In this trial, treatment with oral TU at doses of 80 mg/day and 160 mg/day had no significant effect relative to placebo on IPSS-T, IPSS-V, IPSS-S, IPSS QoL, PSA, or prostate volume. Compared with placebo, treatment with oral TU 240 mg/day led to a statistically significant, but clinically insignificant [Citation7], 1.3-point improvement in IPSS-T score as well as a significant improvement in IPSS QoL. There was a numerical improvement relative to placebo in the number and percentage of patients with clinically significant changes in IPSS-T score in the 240 mg/day group (higher percentage of patients had a clinically significant improvement [≥4-point decrease from baseline] and a lower percentage had a clinically significant deterioration [≥4-point increase from baseline] in IPSS-T at study endpoint compared with placebo), however, the numbers of patients involved with these changes were small. Oral TU 240 md/day had no effect on mean serum PSA or mean prostate volume. The modest improvement in IPSS-T score seen in the present trial with oral TU 240 mg/day appeared to involve more of an improvement in voiding symptoms (IPSS-V; ∼1-point improvement relative to placebo) than an improvement in storage symptoms (IPSS-S; ∼0.3-point improvement).
The present findings are consistent with the results of two smaller, 1-year, placebo-controlled studies with oral TU in aging men. A trial by Wittert et al. in 76 aging, hypogonadal men reported that treatment with oral TU 160 mg/day for one year led to a non-significant numerical improvement relative to placebo in mean IPSS-T score of approximately 1.2 points and had no significant effect on mean serum PSA [Citation9]. Another trial by Bebb et al. in 40 aging, hypogonadal men reported that treatment with oral TU 240 mg/day for one year had no significant effect relative to placebo on urinary symptoms or mean PSA [Citation10]. Serum PSA is a sensitive marker for detecting changes in the prostatic epithelial stimulatory effects of intraprostatic androgens, particularly intraprostatic dihydrotestsoterone (DHT). Intraprostatic DHT and serum PSA levels have been shown to be increased relative to placebo in hypogonadal men receiving TRT [Citation11]. The present trial included serum PSA measurements across multiple time points (Months 1, 3, 6, 9, and 12) in order to monitor closely any changes in prostatic epithelial stimulation. We observed no significant change relative to placebo in mean PSA levels at any of these time points for any of the oral TU doses tested. Taken together, these findings provide physicians consistent and reassuring data concerning the effects of long-term oral TU therapy on urinary function in aging, hypogonadal men.
The question arises regarding the reason for the significant improvement relative to placebo in IPSS QoL score seen with TU 240 mg/day. In the present trial, the mean placebo-adjusted improvement in IPSS-T score observed with TU 240 mg/day was relatively small in magnitude. While there appeared to be an increase relative to placebo in the percentage of patients with clinically significant improvement from baseline in IPSS-T score in the 240 mg/day group, the number of patients with such improvement was small. It therefore seems unlikely that the improvement in IPSS-T alone was responsible for the significant improvement in IPSS QoL score observed in the oral TU 240 mg/day group. The IPSS QoL question assesses how a patient feels about living the rest of their life with their present urinary condition. A patient’s response to such a measure can be altered by their general affect—patients with a more positive affect may be less bothered by ongoing somatic symptoms of mild chronic conditions [Citation12]. In addition to a modest improvement in IPSS-T score, treatment with oral TU 240 mg/day was also shown to be associated with a significant improvement relative to placebo in lean body mass, fat mass, and bone mineral density as well as a numerical improvement in sexual function and Aging Males’ Symptoms (AMS) rating scale score. It is conceivable that these multiple beneficial effects led to a more positive affect and a reduction in the level of bother caused by any ongoing urinary symptoms (which, when they occurred in this trial, were generally mild in intensity), thereby contributing to the improvement seen with oral TU 240 mg/day in IPSS QoL score.
The strengths of this trial include its relatively large size (322 men), long duration (1 year), dose-ranging design, inclusion of IPSS, PSA, and prostate volume measures, and the fact that it enrolled aging men with baseline characteristics frequently encountered in the real-world clinic setting (hypogonadism symptoms, low-normal total testosterone levels, and low free testosterone levels). Limitations of the trial include that it generally enrolled men with normal urinary function–mean baseline IPSS-T score was 5.6.
In summary, this 1-year, placebo-controlled trial in 322 aging men with symptomatic hypogonadism demonstrated that long-term oral TU therapy had no deleterious effects on IPSS-T and was associated with a dose-dependent improvement in IPSS QoL.
Acknowledgements
The authors thank the study investigators and their staff for their assistance in conducting this trial, and the study subjects for their willing participation in this research. The authors also wish to thank Kathleen Newcomb, Jennifer Rotonda, and Kristen Lewis of Merck & Co., Inc., for their assistance in preparing this paper for publication.
Declaration of interest
M.J.G.H.K., A.J.-L and A.G.M. are employees of Merck & Co., Inc., Whitehouse Station, NJ, sponsor of this study. J.M.H.E. and T.B.P.G. were employees of MSD Oss, a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ. P.M.G.B., J.J.L. and E.J.M. have received lecture fees from the sponsor. No other potential conflict of interest relevant to this article was reported. All authors are responsible for the work described in this paper. M.J.G.H.K., A.J.-L., A.G.M., J.M.H.E., T.B.P.G., P.M.G.B., J.J.L. and E.J.M. were involved in at least one of the following: [conception, design, acquisition, analysis, statistical analysis, interpretation of data] and [drafting the manuscript and/or revising/reviewing the manuscript for important intellectual content]. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This study was funded by Organon N.V. (a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ), Oss, The Netherlands (Protocol # 43203).
Clinical trial registry: This trial was registered at www.clinicaltrials.gov under number NCT00434824 (http://www.clinicaltrials.gov/ct2/show/NCT00434824)
References
- Horst HJ, Holtje WJ, Dennis M, et al. Lymphatic absorption and metabolism of orally administered testosterone undecanoate in man. Klin Wochenschr 1976;54:875–9
- Shackleford DM, Faassen WA, Houwing N, et al. Contribution of lymphatically transported testosterone undecanoate to the systemic exposure of testosterone after oral administration of two andriol formulations in conscious lymph duct-cannulated dogs. J Pharmacol Exp Ther 2003;306:925–33
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000;49:1239–42
- Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia: the Measurement Committee of the American Urological Association. J Urol 1992;148:1549–57
- Legros JJ, Meuleman EJ, Elbers JM, et al. Oral testosterone replacement in symptomatic late-onset hypogonadism: effects on rating scales and general safety in a randomized, placebo-controlled study. Eur J Endocrinol 2009;160:821–31
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003;349:2387–98
- Lunenfeld B, Mskhalaya G, Kalinchenko S, Tishova Y. Recommendations on the diagnosis, treatment and monitoring of late-onset hypogonadism in men – a suggested update. Aging Male 2013;16:143–50
- Wittert GA, Chapman IM, Haren MT, et al. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci 2003;58:618–25
- Bebb R, Anawalt B, Wade J, et al. A randomized, double blind, placebo controlled trial of testosterone undecanoate administration in aging hypogonadal men: effects on bone density and body composition. Proceedings of the 83rd Annual Meeting of the Endocrine Society; June 20–23, 2001; Denver, CO
- Marks LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA 2006;296:2351–61
- Watson D, Pennebaker JW. Health complaints, stress, and distress: exploring the central role of negative affectivity. Psychol Rev 1989;96:234–54