Abstract
Type 2 diabetes mellitus (T2DM) is often associated with obesity and subnormal serum testosterone (T) levels. Until 5 years ago there was no indication that men with type 1 diabetes mellitus (T1DM) had subnormal serum T. But recent studies indicate that about 10% of men with T1DM suffer from hypogonadism, as a rule aged men and men with obesity. While hypogonadal men with T2DM benefit from normalization of their serum T, this has not been investigated in men with T1DM. Nine men with T1DM, erectile dysfunction and hypogonadism (total testosterone ≤ 12 nmol/L) received testosterone replacement therapy (TRT). In seven men TRT was intermitted: one man with prostate malignancy and six men because of problems of reimbursement. Incidentally, this provided an opportunity to monitor the effects of withdrawal and of the reinstatement of TRT. In all men, glycemic control (serum glucose and HbA1c), weight, waist circumference, lipid profiles and erectile function improved upon TRT. The seven men whose TRT was intermitted showed a deterioration which improved again upon reinstatement of TRT. The data suggest that aging and obese men with T1DM might have subnormal T levels and that their glycemic control, lipid profiles and erectile function might benefit from TRT.
Introduction
Of late there has been some attention to the issue of low serum testosterone levels in men suffering from type 1 diabetes mellitus (T1DM). Essentially, hypogonadal patients with T1DM share characteristics with type 2 diabetes mellitus (T2DM): they are abdominally obese and they are older [Citation1–6], but testosterone deficiency is more severe in men with T2DM. Insulin requirements and serum triglycerides also predict hypogonadotropic hypogonadism in men with T1DM [Citation6]. Upon a search of the literature, there were no reports on the effects of normalizing serum testosterone (T) in men with T1DM with subnormal serum testosterone [Citation7]. In the present report, some first observations are provided in nine men. These nine men belonged to a cumulative, prospective, registry study of 262 hypogonadal men (total testosterone ≤ 12 nmol/L) receiving TRT. They had sought medical consultation because of erectile dysfunction. The present study was not specifically designed to monitor the effects of TRT on variables of glycemic control and lipid patterns. In seven of these nine men there was an intermission in TRT. In one man because of a prostate malignancy and in six men because of problems of cost reimbursements for testosterone treatment. Incidentally, this provided an opportunity to monitor the effects of withdrawal of TRT and of the reinstatement of TRT.
Subjects and methods
Since 2004, a single center, cumulative, prospective, ongoing registry study of 262 hypogonadal men (total testosterone ≤ 12 nmol/L) receiving testosterone undecanoate (TU) 1000 mg injections every 12 weeks following an initial 6-week interval is being conducted. Among a variety of comorbidities present at baseline, nine men (3.4%) had a diagnosis of type 1 diabetes mellitus (T1DM) from the initiation of the study. This subgroup of nine patients was separately analyzed.
Patients' age at baseline was between 52 and 68 years. All men were followed for at least 6 years, three men for 9 years, and five men were followed for 10 years. This is a result of the registry design where patients are enrolled at different time points and does not represent drop-outs. TRT was temporarily interrupted for 1 year in a patient diagnosed with prostate cancer, and for 1.5 to 2 years in six men due to reimbursement issues. In all seven men, TRT was resumed for a period of 1 to 2.5 years (end of observation time of this analysis). Anthropometric parameters, such as body weight, body mass index (BMI), waist circumference (WC), total testosterone, SHBG, fasting glucose, HbA1c, total cholesterol, triglycerides, LDL, HDL were measured every 6 months after an overnight fast.
Measurements of anthropometric parameters were performed at baseline (height, weight, WC) and at each other visit (weight, WC). Also the IIEF-EF consisting of six questions with a maximum score of 30 was assessed then. Blood samples were drawn at each or each other visit in the morning, prior to the next injection of testosterone. Therefore, testosterone levels were trough levels at the end of an injection interval. WC was measured midway between lowest rib and the uppermost border of the right iliac crest. T was measured in a single laboratory by standard laboratory measurement as described elsewhere [Citation8]. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss [Citation8]. Ethical guidelines as formulated by the German “Ärztekammer” (the German Medical Association) for observational studies in patients receiving standard treatment were followed. Patients had consented before starting testosterone administration that their data might provide material for scientific studies, but were asked again before the analysis of the specific data for this publication. After receiving an explanation regarding the nature and the purpose of the study, all subjects consented to be included in the research of the data of their treatment protocol.
Statistical analysis
Statistical analyses were performed for the first 6 years. For continuous variables, the mean, median, standard deviation, range, minimum, maximum, and sample size for the overall sample and various groups were reported at each time point. For categorical variables, the frequency distribution was reported. We tested the hypotheses regarding change in outcome scores across the study period by fitting a linear mixed effects model to the data. Time (to indicate follow-up interviews) was included as fixed effect in the model. A random effect was included in the model for the intercept. Estimation and test of change in scores were determined by computing the differences in least square means at baseline versus the score at each follow-up interview.
Results
Baseline characteristics of the patients are presented in . Mean patient age at baseline was 60.56 ± 6.89 years (minimum: 52, maximum: 68 years).
Table 1. Baseline characteristics of 9 hypogonadal men with diabetes mellitus type 1 receiving testosterone treatment.
Mean total testosterone increased from 7.5 ± 1.63 to 16.7 ± 5.52 nmol/L after 1 year and then stabilized between 18.8 and 20 nmol/L until intermission. During intermission, testosterone dropped to hypogonadal levels, in some patients below baseline. Mean calculated free testosterone at baseline was 152.9 ± 72.15 pmol/L.
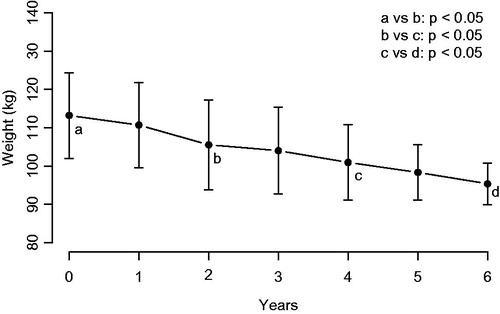
Seven patients were obese (two with excessive obesity) and two were overweight. Mean weight decreased from 113.22 ± 17.12 to 95.39 ± 8.28 kg after 6 years (). In all obese patients while on TRT, weight decreased by 8 to 41 kg. One of the overweight patients (baseline BMI: 25.6 kg/m2) did not experience weight loss, the other overweight patient (follow-up: 6 years) lost 6 kg. During TRT interruption, weight regain ranged from 1 to 9 kg. After resuming TRT, weight decreased again by 1 to 13 kg ().
Table 2. Glycemic control of nine hypogonadal men with type 1 diabetes mellitus receiving testosterone treatment (TRT).
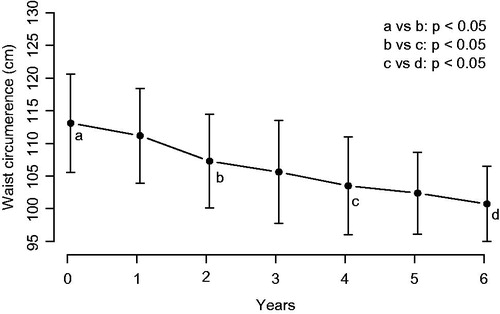
During the first 6 years, mean WC decreased from 113.11 ± 11.5 to 100.72 ± 8.83 cm (). WC decreased in all patients from 3 to 25 cm. During intermission of TRT in seven men, waist circumference increased in all but one patient by 1 to 8 cm. After resumption of TRT, waist circumference remained stable in two and decreased again by 2 to 8 cm in five men ().
Figure 2. Waist circumference (cm) in nine men with type 1 diabetes receiving treatment with testosterone.
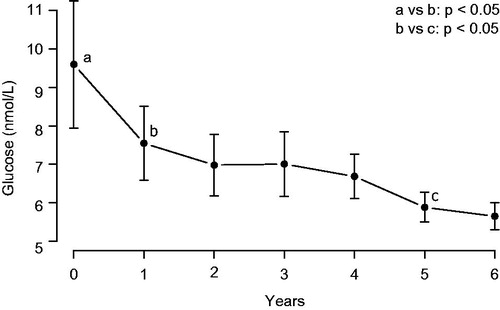
Baseline fasting glucose was 9.54 ± 2.81 mmol/L (minimum: 4.4, maximum: 12.9) and dropped under TRT during the first 6 years to 5.16 ± 0.6 mmol/L (minimum: 4.02, maximum: 5.97) (). Serum glucose increased during intermission of TRT to between 5.9 and 11.0 mmol/L, and decreased again to between 3.9 and 6.2 mmol/L upon resuming TRT ().
Figure 3. Serum glucose (mmol/L) in nine men with type 1 diabetes receiving treatment with testosterone.
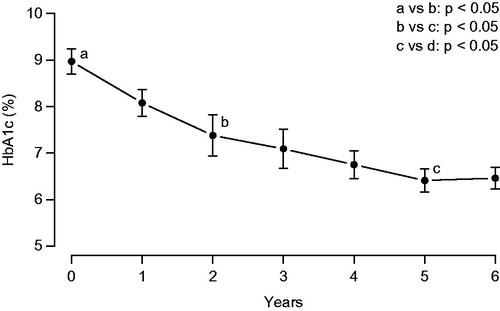
During the first 6 years, HbA1c ranged from 8.4 to 9.7% at baseline (mean: 8.97 ± 0.42%). Upon TRT, HbA1c decreased to values between 5.7 and 6.9% (). HbA1c increased to values between 7.2 and 9.1% during the intermission of TRT and declined again upon resumption of TRT to values between 5.8 and 6.9% ().
During the entire observation time accumulating to 963 patient-months (80 patient-years), there were no drop-outs, no major adverse cardiovascular event (MACE), and no urological event such as acute urinary retention.
Discussion
It is well established that T2DM is associated with low serum testosterone levels and that a bidirectional relationship exists between testosterone and obesity: obesity is associated with low serum testosterone and, vice versa, low serum testosterone induces obesity and its sequels, such as T2DM, dyslipidemia and cardiovascular disease. Several studies document the beneficial effects of normalizing serum testosterone in men with the metabolic syndrome: obesity, T2DM and cardiovascular disease [Citation9–14]. Much less is known about the association of T1DM and testosterone. Early studies indicated that, in contrast to men with T2DM, men with T1DM had normal serum T [Citation15]. But more recent studies show that subcategories of men with T1DM have subnormal serum testosterone, particularly when these men are obese and aged [Citation1–6], in fact similar to findings in men with T2DM. Its prevalence has been estimated to be 10% [Citation1,Citation6]. Grossmann et al. [Citation3] found a higher prevalence of hypogonadism based on free testosterone in T1DM of 20.3% with a cut-off of 230 pmol/L of calculated free T. In our study, all but one man had a free testosterone below that threshold.
In this paper, we analyzed the effects of TRT in a subgroup of men with a specific comorbidity from a cumulative, prospective, registry study of 262 hypogonadal men (total testosterone ≤ 12 nmol/L) receiving TRT. Nine men had a previous diagnosis of T1DM and suffered from erectile dysfunction, the reason why they were seeking urological consultation. The present study was designed to monitor long-term effectiveness and tolerability of TRT with testosterone undecanoate injections. It was not specifically designed to monitor the effects of TRT in hypogonadal men with T1DM or on variables of glycemic control and lipid patterns. Therefore, our results in this specific subgroup were unexpected. The results could be coincidental observations, but are nevertheless encouraging to undertake larger, better designed studies. In seven of these nine men there was an intermission in TRT, in one man because of a prostate malignancy and in six men because of problems of cost reimbursements for testosterone treatment. Incidentally, this provided an opportunity to monitor the effects of withdrawal of testosterone and of the reinstatement of TRT. Upon TRT, we observed an improvement in glycemic control: both fasting glucose and HbA1c declined upon TRT, worsened during the intermission of TRT and improved again during reinstatement of TRT. Anthropometric variables (body weight, BMI and waist circumference) followed a similar course. This accidental observation lends credence to the assumption that TRT was a factor in the improvement of the diabetic condition of the patients.
A limitation of this study is the small number of patients with T1DM. Another limitation lies in the fact that no information on changes of concomitant medications was collected. All men were treated for their T1DM by their respective family physicians/internists and were on insulin therapy. However, potential changes in dosage were not recorded. They primarily had consulted the urologist with complaints of erectile dysfunction. Although this study, an observation on nine men, was not designed to study the effects of TRT on glycemic control of T1DM and erectile function, the data provide credible evidence that in men with T1DM serum testosterone should be measured when these men are obese and aged. Our preliminary data indicate that restoring serum testosterone to normal levels may produce a beneficial effect on the management of glycemic control with a simultaneous improvement of anthropometric patterns and lipid profiles. Interestingly, a very recent study indicated that improved glycemic control may not be an expected outcome of weight loss after bariatric surgery in patients with T1DM [Citation16]. Extrapolated to our study it would seem that the beneficial effects of testosterone on glycemic control are rather the effects of testosterone itself than of the associated weight reduction with testosterone treatment. This intervention might reduce cardiovascular risks associated with T1DM.
The above findings, however, should be more solidly demonstrated in better designed and larger studies.
Acknowledgements
Farid Saad, Louis Gooren and Gheorghe Doros contributed to the conception and design of the case study. Drs A.Yassin and Y. Almehmadi were pivotal in data acquisition. Gheorghe Doros, Farid Saad and Louis Gooren, have analyzed and interpreted the data. All authors have approved the final version for submission.
Declaration of interest
Dr A. Yassin received compensation for data entry from Bayer Pharma. Dr Doros received a honorarium for statistical analyses from Bayer Pharma.
Farid Saad is an employee of Bayer Pharma AG, the manufacturer of testosterone undecanoate, the androgen used in this study. The other authors declare no conflict of interests.
References
- Holt SK, Lopushnyan N, Hotaling J, et al. Prevalence of low testosterone and predisposing risk factors in men with type 1 diabetes mellitus: findings from the DCCT/EDIC. J Clin Endocrinol Metab 2014;99:E1655–60
- Ng Tang Fui M, Hoermann R, Cheung AS, et al. Obesity and age as dominant correlates of low testosterone in men irrespective of diabetes status. Andrology 2013;1:906–12
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008;93:1834–40
- Biswas M, Hampton D, Newcombe RG, Rees DA. Total and free testosterone concentrations are strongly influenced by age and central obesity in men with type 1 and type 2 diabetes but correlate weakly with symptoms of androgen deficiency and diabetes-related quality of life. Clin Endocrinol (Oxf) 2012;76:665–73
- Chandel A, Dhindsa S, Topiwala S, et al. Testosterone concentration in young patients with diabetes. Diabetes Care 2008;31:2013–17
- Chillaron JJ, Fernandez-Miro M, Albareda M, et al. Age, insulin requirements, waist circumference, and triglycerides predict hypogonadotropic hypogonadism in patients with type 1 diabetes. J Sex Med 2015;12:76–82
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015;18:5–15
- Yassin A, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clin Obes 2013;3:73–83
- Haider A, Saad F, Doros G, Gooren L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational study. Obes Res Clin Pract 2014;8:e339–49
- Saad F, Aversa A, Isidori AM, Gooren LJ. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: a review. Curr Diabetes Rev 2012;8:131–43
- Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with “diabesity”: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol 2014;2014:683515. doi: 10.1155/2014/683515
- Corona G, Rastrelli G, Monami M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol 2013;168:829–43
- Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring) 2013;21:1975–81
- Francomano D, Lenzi A, Aversa A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol 2014;2014:527470. doi: 10.1155/2014/527470
- Tomar R, Dhindsa S, Chaudhuri A, et al. Contrasting testosterone concentrations in type 1 and type 2 diabetes. Diabetes Care 2006;29:1120–2
- Maraka S, Kudva YC, Kellogg TA, et al. Bariatric surgery and diabetes: implications of type 1 versus insulin-requiring type 2. Obesity (Silver Spring) 2015;23:552--7