Abstract
We investigated the fracture risk after androgen deprivation therapy (ADT) for prostate cancer in the Chinese population. All Chinese prostate cancer patients who were treated primarily by radical prostatectomy or radiotherapy, with or without further ADT, from year 2000 to 2009 were reviewed. We compared the fracture risk in patients who were given ADT (ADT group) with those who were not given any ADT (non-ADT group). Potential risk factors including age, diabetes mellitus, hypertension, hyperlipidemia, ischemic heart disease and performance status were reviewed. The fracture risk was analyzed with Kaplan–Meier and multivariate Cox regression analyses. Our cohort consisted of 200 patients in the non-ADT group and 252 patients in the ADT group. The ADT group was shown to have higher fracture risk (p = 0.036) upon Kaplan–Meier analysis. Upon multivariate Cox regression analyses, diabetes mellitus (HR 4.39, 95% CI 1.08–17.83, p = 0.039), poor performance status (HR 3.14, 95% CI 1.24–8.00, p = 0.016) and the use of ADT (HR 4.89, 95% CI 1.03–23.17, p = 0.045) were associated with increased fracture risk. In conclusion, the fracture risk should be considered while deciding on ADT in Chinese men, especially in diabetic patients with poor performance status.
Introduction
While androgen deprivation therapy (ADT) is often given to patients with advanced or metastatic prostate cancer, there has been increasing use of ADT in patients with localized disease [Citation1]. The use of adjuvant ADT has been shown to prolong survival in node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy [Citation2], and in locally advanced prostate cancer following external irradiation [Citation3]. However, ADT is associated with many side effects [Citation4,Citation5], and one should expect deterioration in sexual function after the use of ADT [Citation6]. The use of ADT may also lead to major adverse events including diabetes mellitus [Citation7–10], skeletal fracture [Citation11–13], myocardial infarction [Citation7–9] and stroke [Citation7–9].
Bone health is an important aspect in prostate cancer management. The use of ADT will result in suppression of testosterone level, and osteogenesis may be impaired due to inhibition of the Wnt signaling pathway [Citation14]. Moreover, serum testosterone is normally aromatized to estradiol. While estrogen protects against bone demineralization [Citation15], ADT may affect bone turnover through suppression of the estrogen level. The use of ADT may reduce bone mineral density [Citation16,Citation17] and induce micro-architectural changes in cortical bone [Citation18], which in turn lead to increased fracture risk. The development of bone fracture not only causes pain, it may necessitate surgery and lead to decline in quality of life in long run. The occurrence of bone fracture has been shown to have a negative impact on overall survival in patients with prostate cancer [Citation19]. The development of ADT-related adverse events is detrimental and may potentially outweigh the benefits of ADT.
There were three large-scale population-based studies [Citation11–13] investigating the fracture risk after ADT in men with prostate cancer. All of them demonstrated increased fracture risk after ADT, but the results were largely based on Caucasian data [Citation11–13]. The association between ADT and fracture in the Chinese population remained largely unknown. To our knowledge, this is the first study investigating the association between ADT and fracture in the Chinese population.
Methods
This was a retrospective cohort study conducted in a university hospital in Hong Kong. All prostate cancer patients who were treated by radical prostatectomy or radiotherapy, with or without further ADT, at our hospital from year 2000 to 2009 were reviewed. Patients who were treated primarily by ADT and those who had bone metastases upon primary treatment were excluded. Patients who were not Chinese in ethnic origin were also excluded. The research protocol of our study was reviewed and approved by our local institutional ethics committee.
Clinicopathological data including baseline prostate-specific antigen (PSA) level, Gleason score, clinical T-stage, form of primary treatment, form of ADT, ADT treatment setting, ADT treatment approach and duration of ADT were recorded. Clinical variables that may affect the risk of ADT-related fracture including age, pre-existing medical conditions including diabetes mellitus, hypertension, hyperlipidemia and ischemic heart disease, and the ECOG performance status [Citation20] were reviewed. These data were retrieved from medical records of the patients. Prostate-specific antigen level was measured using a Roche Cobas e601 system (Roche Diagnostic Corp., Indianapolis, IN) with standardization against the WHO 96/670 reference standard. The intra-assay coefficient of variation was 1.2–1.7%, and the inter-assay coefficient of variation was 2.4–3.2%.
The primary outcome measured is new fracture event after ADT. We compared the fracture risk in patients who received further ADT (ADT group) with those who did not receive any ADT (non-ADT group). We compared the potential risk factors of fracture between the two groups. Independent sample t test was used for age, and chi-square test was used for diabetes mellitus, hypertension, hyperlipidemia, pre-existing ischemic heart disease and ECOG performance status. The fracture risk was analyzed using Kaplan–Meier analysis, with the significance being determined by log-rank test. Further multivariate Cox regression analyses were performed to adjust for other potential risk factors of ADT-related fracture. p value of <0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY).
Results
A total of 452 patients were treated primarily with radical surgery or radiotherapy from year 2000 to 2009, which consisted of 200 patients in the non-ADT group and 252 patients in the ADT group. The median follow-up was 72.9 months. The overall mean age was 69.0 ± 6.3 years (range from 45.5 to 83.1 years) in our cohort. The mean age was 68.2 ± 5.9 years in the non-ADT group and 69.5 ± 6.5 years in the ADT group (p = 0.031). The median baseline PSA level was 9.75 ng/ml in the non-ADT group and 27.1 ng/ml in the ADT group. The majority of the patients had Gleason score pattern of ≤6 in the non-ADT group (71%), while it was relatively equally distributed in the ADT group (34.2% had Gleason score pattern of ≤6, 32.9% of 7 and 32.9% of 8–10). The majority of the patients had clinical T-stage 2 disease in both the non-ADT (69.8%) and the ADT groups (41.3%). Concerning the primary form of treatment, 68.5% of the non-ADT group had radical surgery and 73.4% of the ADT group had radiotherapy. The two groups differed significantly in terms of their age, baseline PSA, Gleason score, clinical T-stage and the form of primary treatment (p < 0.05). In the ADT group, most of the patients (81.8%) had gonadotropin-releasing hormone (GnRH) agonist as the form of ADT. Androgen deprivation therapy was given in an adjuvant setting (74.9%) and mostly in a continuous approach (96.0%) in the majority of the patients. The mean duration of ADT was 40.3 ± 34.1 months ().
Table 1. The clinicopathological data of the cohort.
Concerning the potential risk factors of ADT-related fracture, age was significantly higher in the ADT group (p = 0.031). There was no significant difference in their pre-existing medical conditions including diabetes mellitus, hypertension, hyperlipidemia and ischemic heart disease (p > 0.05). The majority of the patients had good ECOG performance status of 0 (89.0% in the non-ADT group and 88.1% in the ADT group), and no significant difference was noted between the two groups (p > 0.05) ().
Table 2. Comparison of the clinical variables that may affect the risk of ADT-related fracture.
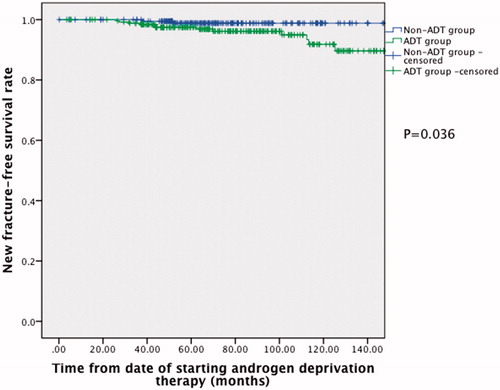
In the whole cohort, 14 patients developed new fracture during the follow-up period; 2 patients (1.0%) in the non-ADT group and 12 patients (4.8%) in the ADT group. Upon Kaplan–Meier analysis (), the ADT group was shown to have increased fracture risk (p = 0.036). Upon multivariate Cox regression analyses (), diabetes mellitus (HR 4.39, 95% CI 1.08–17.83, p = 0.039), poor ECOG performance status (HR 3.14, 95% CI 1.24–8.00, p = 0.016) and the use of ADT (HR 4.89, 95% CI 1.03–23.17, p = 0.045) were significantly associated with increased fracture risk. Although there was a significant difference in mean age between the two groups, age was not associated with increased fracture risk upon multivariate analyses (HR 1.03, 95% CI 0.95–1.12, p > 0.05).
Table 3. Multivariate Cox regression analyses for fracture risk.
Discussion
In our study, a total of 452 Chinese prostate cancer patients who underwent radical surgery or radiotherapy, with (ADT group) or without further ADT (non-ADT group), during a 10-year period were included. This represents a cohort of non-metastatic disease, and our study investigated the risk of fracture, or more specifically osteoporotic fracture, in patients receiving ADT. The ADT group was shown to have higher fracture risk (p = 0.036) upon Kaplan–Meier analysis. Diabetes (HR 4.39, 95% CI 1.08–17.83, p = 0.039), poor ECOG performance status (HR 3.14, 95% CI 1.24–8.00, p = 0.016) and the use of ADT (HR 4.89, 95% CI 1.03–23.17, p = 0.045) were significantly associated with increased fracture risk upon multivariate Cox regression analyses.
The use of ADT has been shown to cause significant reduction in bone mineral density [Citation16,Citation17,Citation21]. While the majority of the literature was based on Caucasians, there is a lack of data regarding the association of ADT and bone mineral density in the Asian population. Two studies from the same Japanese research group investigated the effects of ADT on bone mineral density in ADT-treated and hormone-naïve Japanese men. The first study is a cross-sectional study which included 58 ADT-treated and 43 hormone-naïve Japanese prostate cancer patients [Citation22]. The differences in bone mineral density values measured at the lumbar spine, total hip and femoral neck between the two groups of patients did not reach statistical significance. The cohort was then extended to include 113 ADT-treated and 88 hormone-naïve patients [Citation23]. The ADT-treated patients were shown to have significantly lower bone mineral density values than the hormone-naïve patients. Interestingly, both studies showed that Japanese prostate cancer patients appeared to have much lower rates of osteoporosis in both hormone-naïve (ranging from 2.3 to 4.5%) and ADT-treated (ranging from 8.6 to 12.1%) patients, when compared to the Caucasian population (35.4% in hormone-naïve patients and 42.9% in patients after receiving 2 years of ADT) [Citation24]. This may be due to the underlying genetic and physiological differences between different ethnicities, but further studies will be needed to address these issues.
To date, there were three large-scale population-based studies [Citation11–13] demonstrating increased fracture risk after the use of ADT in men with prostate cancer. Two of the studies [Citation11,Citation13] included heterogeneous groups of patients with non-metastatic and metastatic diseases. While ADT is more often indicated in metastatic disease, the observed increased fracture risk after ADT may be accounted by the occurrence of pathological fracture rather than osteoporotic fracture. The study by Smith et al. [Citation12] demonstrated increased fracture risk in a claims-based cohort of 11 661 men with non-metastatic prostate cancer. The cohort being studied was similar to that of our study. However, only a limited number of pre-existing medical conditions were adjusted in their analyses, which may potentially affect the accuracy and reliability of the results. While the majority of the literature was based on Caucasians [Citation11–13], the association of ADT and its adverse events in the Chinese population remained largely unknown. In this study, we investigated the fracture risk after ADT for prostate cancer in our local Chinese cohort.
Our study showed that the use of ADT was associated with increased fracture risk upon both Kaplan–Meier (p = 0.036) and multivariate Cox regression analyses (HR 4.89, 95% CI 1.03–23.17, p = 0.045). The bone remodeling process involves bone formation and resorption, which are mediated by the actions of osteoblasts and osteoclasts [Citation25]. The function of osteoclasts is regulated by various factors including the receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin [Citation26], whereas the Wnt signaling pathway is also essential for normal osteogenesis [Citation27]. The use of ADT may result in suppression of both testosterone and estrogen levels, and the alteration of hormonal balances may result in disturbances in the bone remodeling process. Sclerostin, an inhibitor of the Wnt signaling pathway, was shown to be elevated in patients receiving ADT [Citation14]; the significant negative correlation between sclerostin and testosterone may result in impaired osteogenesis due to inhibition of the Wnt signaling pathway. Moreover, serum testosterone is normally aromatized to estradiol. Since estrogen protects against bone demineralization [Citation15], the suppression of estrogen may reduce bone mineral density [Citation16,Citation17] and induce micro-architectural changes in cortical bone [Citation18], and therefore leading to increased fracture risk. As the number of patients having bilateral orchiectomy as the only form of ADT (25 patients), and the number of events of new fracture in the cohort (14 events) were small, we did not sub-stratify the form of ADT in our analyses. Whether there is any difference in the fracture risk between bilateral orchiectomy and GnRH agonist is an interesting question that should be explored in future studies.
A poor ECOG performance status (HR 3.14, 95% CI 1.24–8.00, p = 0.016) was associated with increased fracture risk. Since patients with poor ECOG performance status were more fragile and were of poorer general condition, they were at higher fracture risk. Whether regular mobilization and exercise would reduce fracture risk in prostate cancer patients receiving ADT is an interesting issue that should be explored. Regular exercise may also improve prostate cancer patients’ psychology along the course of cancer treatment [Citation28].
Interestingly, diabetes mellitus (HR 4.39, 95% CI 1.08–17.83, p = 0.039) was also a significant risk factor of fracture. There are several mechanisms that may account for this association. Firstly, bone remodeling may be impaired in diabetic patients, as shown by reduction in both markers of bone formation and bone resorption when compared to healthy subjects [Citation29–32]. Serum sclerostin was also found to be significantly increased in diabetic patients [Citation31,Citation32], which may affect bone formation through inhibition of the Wnt signaling pathway. Secondly, diabetic patients may experience increased falling frequency [Citation33]. This may be secondary to both diabetic retinopathy causing visual impairments and diabetic neuropathy causing sensory, motor and autonomic dysfunction. Frequent hypoglycemia after anti-diabetic medications may also predispose diabetic patients to increased fall risk. Thirdly, some anti-diabetic medications may affect bone fragility. Thiazolidinediones have been shown to have a negative effect on bone quality by suppressing the differentiation of mesenchymal stem cells into osteoblasts and favoring the differentiation into adipocytes instead [Citation34,Citation35]. This may affect bone fragility, hence leading to increased fracture risk in diabetic patients.
To our knowledge, this is the first study investigating the association between ADT and fracture in the Chinese population. Fracture may cause mobility problems and significantly affect the quality of life in long run. The occurrence of fracture may also adversely affect survival in prostate cancer patients [Citation19]. Our results showed that ADT is associated with increased fracture risk and should be an important factor to consider while deciding on the initiation of ADT, especially in diabetic patients with poor performance status. The use of FRAX and Garvan nomogram may also help predict the occurrence of fracture in men with osteoporosis [Citation36]. Additional attention should be made if ADT has to be given to those patients who are at high fracture risk.
There are several limitations in our study. Firstly, this study is retrospective in nature. Potential confounding factors including patients’ body mass index and their pre-existing medical conditions were not routinely checked before primary treatment, and the accuracy of our results may be affected. The sex hormone levels were not routinely checked or monitored, hence the association between the levels of sex hormones and the fracture risk could not be determined by our results. There is a lack of data regarding the bone mineral density in our cohort before and after the intended treatment. Secondly, since patients who received radical surgery or radiotherapy had localized disease with relatively good premorbid status, the number of new events of fracture is small and the results have to be interpreted with caution. Thirdly, the use of medications that may improve osteoporosis such as bisphosphonates was unknown and the results may be affected.
In summary, diabetes mellitus, poor ECOG performance status and the use of ADT increased fracture risk in Chinese men treated for prostate cancer. There is a lack of data regarding the effects of ADT on bone health in the Chinese population. Further prospective studies will be needed to investigate the effects of ADT on bone mineral density and the associations between ADT, osteoporotic fracture and, more importantly, survival in Chinese prostate cancer patients.
Declaration of interest
The authors report no declarations of interest.
References
- Shahinian VB, Kuo YF, Freeman JL, et al. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer 2005;103:1615–24
- Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 2006;7:472–9
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002;360:103–6
- Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA 2005;294:238–44
- Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin 2002;52:154–79
- Lebret T, Culine S, Davin JL, et al. Quality of life of 1276 elderly patients with prostate cancer, starting treatment with a gonadotropin-releasing hormone agonist: results of a French observational study. Aging Male 2014;17:87–93
- Keating NL, O’Malley A, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 2012;104:1518–23
- Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 2010;102:39–46
- Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006;24:4448–56
- Teoh JY, Chiu PK, Chan SY, et al. Risk of new-onset diabetes after androgen deprivation therapy for prostate cancer in the asian population. J Diabetes 2014. doi: 10.1111/1753-0407.12226. [Epub ahead of print]
- Dickman PW, Adolfsson J, Astrom K, Steineck G. Hip fractures in men with prostate cancer treated with orchiectomy. J Urol 2004;172:2208–12
- Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol 2005;23:7897–903
- Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 2005;352:154–64
- Garcia-Fontana B, Morales-Santana S, Varsavsky M, et al. Sclerostin serum levels in prostate cancer patients and their relationship with sex steroids. Osteoporos Int 2014;25:645–51
- Grossmann M, Cheung AS, Zajac JD. Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Pract Res Clin Endocrinol Metab 2013;27:603–16
- Basaria S, Lieb J II, Tang AM, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 2002;56:779–86
- Galvao DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int 2008;102:44–7
- Hamilton EJ, Ghasem-Zadeh A, Gianatti E, et al. Structural decay of bone microarchitecture in men with prostate cancer treated with androgen deprivation therapy. J Clin Endocrinol Metab 2010;95:E456–63
- Oefelein MG, Ricchiuti V, Conrad W, Resnick MI. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol 2002;168:1005–7
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55
- Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy X-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol 2002;167:2361–7; discussion 7
- Wang W, Yuasa T, Tsuchiya N, et al. Bone mineral density in Japanese prostate cancer patients under androgen-deprivation therapy. Endocr Relat Cancer 2008;15:943–52
- Yuasa T, Maita S, Tsuchiya N, et al. Relationship between bone mineral density and androgen-deprivation therapy in Japanese prostate cancer patients. Urology 2010;75:1131–7
- Morote J, Morin JP, Orsola A, et al. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology 2007;69:500–4
- Planas Morin J, Morote Robles J. Skeletal complications of ADT: disease burden and treatment options. Asian J Androl 2012;14:670–5
- Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999;397:315–23
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 2005;8:739–50
- Wright-St Clair VA, Malcolm W, Keogh JW. The lived experience of physically active older prostate cancer survivors on androgen deprivation therapy. Aging Male 2014;17:57–62
- Oz SG, Guven GS, Kilicarslan A, et al. Evaluation of bone metabolism and bone mass in patients with type-2 diabetes mellitus. J Natl Med Assoc 2006;98:1598–604
- Gaudio A, Privitera F, Battaglia K, et al. Sclerostin levels associated with inhibition of the Wnt/beta-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J Clin Endocrinol Metab 2012;97:3744–50
- Ardawi MS, Akhbar DH, Alshaikh A, et al. Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone 2013;56:355–62
- Gennari L, Merlotti D, Valenti R, et al. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab 2012;97:1737–44
- de Waard EA, van Geel TA, Savelberg HH, et al. Increased fracture risk in patients with type 2 diabetes mellitus: an overview of the underlying mechanisms and the usefulness of imaging modalities and fracture risk assessment tools. Maturitas 2014;79(3):265–74
- Grey A. Skeletal consequences of thiazolidinedione therapy. Osteoporos Int 2008;19:129–37
- Benvenuti S, Cellai I, Luciani P, et al. Rosiglitazone stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells. J Endocrinol Invest 2007;30:RC26–30
- Pluskiewicz W, Adamczyk P, Franek E, et al. FRAX calculator and Garvan nomogram in male osteoporotic population. Aging Male 2014;17:174–82