Abstract
A significant amount of epidemiological evidences have underlined an emerging link between metabolic syndrome (MetS) and lower urinary tract symptoms (LUTS) secondary to benign prostatic enlargement a (BPE). We aimed to assess the connections between LUTS and MetS with its components. Meta-analysis were conducted to determine the mean differences (MD) and confidence intervals of IPSS total score, IPSS-voiding, IPSS-storage and prostate volume (PV) in patients with or without MetS. Ln(odds-ratio) were calculated to estimate the risk of having moderate-to-severe LUTS (IPSS ≥ 8). Nineteen studies were identified as eligible for this systematic review, with a total of 18,476 participants, including 5554 (30.06%) with and 12 922 (69.94%) without MetS. Pooled analysis did not demonstrate significant MD of IPSS, IPSS-voiding and IPSS-storage in men with or without MetS but PV was significantly different (MD = 2.18; p = 0.03). Presence of MetS was not significantly associated with moderate-to-severe LUTS (odds ratio = 1.13; p = 0.53) and only altered serum triglycerides and diabetes were associated with this risk. The association between MetS and LUTS/BPE remain unclear and further observational studies in a population with metabolic disorders should be conducted in order to address it's potential role in determining LUTS/BPE.
Introduction
Metabolic syndrome (MetS) is a widespread epidemic disease that included a cluster metabolic alteration with a high socio-economic impact, due to its association with increased morbidity and mortality.
A significant amount of epidemiological evidences have underlined an emerging link between MetS, benign prostatic enlargement (BPE) secondary to benign prostatic hyperplasia (BPH) and related lower urinary tract symptoms (LUTS) [Citation1–4].
Among these features, central obesity, lipidic disorder and hyperinsulinemia represent the trigger causes of MetS and related pathological conditions. These alterations include increase in the activity of the sympathetic nervous system and muscle tone of the prostate, resulting in more severe LUTS independently from prostate enlargement [Citation5–7]. Among MetS features, reduced HDL and increased triglyceride levels were significantly related to higher prostatic inflammation by secreting IL-8 in response not only to oxidized LDL, but also to insulin [Citation8], indicating that different MetS features could synergistically boost inflammation and tissue-remodeling in BPH/LUTS [Citation5,Citation9,Citation10]. In a recent meta-analysis, authors found that MetS-induced differences in prostate volumes were greater in patients with metabolic disorders. Hence, obese, dyslipidaemic and aged patients were more at risk of having MetS as a determinant of their increased prostate size [Citation1].
However, besides previous pathological pathways, the presence of BPE and the observation of MetS should be not directly attributable to the presence of more severe LUTS [Citation11]. In fact, discordant results have rising the hypothesis of ethnic disparities about the connection between MetS and LUTS/BPE [Citation12,Citation13].
The aim of this systematic review and meta-analysis was to assess the connections between LUTS/BPE and MetS with its components.
Methods
Systematic literature search
This analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines [Citation14]. We performed a systematic literature search of PubMed, EMBASE, Cochrane, and Academic One File databases using Medical Subject Headings (MeSH) indexes, keyword searches, and publication types until December 2014. The search was limited to English-Language articles. The search terms included “prostate“, “benign prostatic hyperplasia“, “benign prostatic enlargement“, “metabolic syndrome“, “prostate volume“, “insulin resistance“, “obesity, “hypertension“, “triglycerides“, “cholesterol”, “lower urinary tract symptoms”. We also searched reference lists of relevant articles. We tried to contact all corresponding authors when data were found to be missing.
We defined MetS according to the USA National Cholesterol Education Program – Adult Treatment Panel III (NCEP-ATPIII), which requires at least three of the following five components: central obesity (waist circumference of >94 cm), elevated triglycerides (≥1.7 mmol/L or 150 mg/dL), elevated blood pressure (≥130/85 mmHg), elevated fasting glucose (≥6.1 mmol/L or 110 mg/dL) and reduced HDL cholesterol (<1.03 mmol/L or 40 mg/dL). Previous diagnoses of hypertension and type-2 diabetes mellitus were included as evidence of raised blood pressure or fasting glucose. We also included studies based on the revised MetS criteria proposed by the International Federation of Diabetes and the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI criteria). The latter essentially differs in its reduced threshold of hyperglycemia of 6.0 mmol/L (or 100 mg/dL) and in considering possible ethnic differences in the waist circumference (WC) threshold (≥90 cm for South-Asians and ≥94 cm for Caucasian) [Citation1].
Eligible studies included published journal articles that provided quantitative data on LUTS assessed by the validated International Prostate Symptom Score (IPSS) and prostate volume (PV).
Study selection
Citation lists of retrieved articles were screened manually to ensure sensitivity of the search strategy. References of the included papers were hand searched to identify other potential relevant studies. Studies were reviewed by two independent reviewers (G.I.R. and D.U.); differences in opinion were discussed in consultation with the last author (G.M.).
Data were extracted independently by two reviewers. Discrepancies for inclusion between the investigators were resolved by discussion or further consultation with a third author.
The quality of these eligible citations was assessed using Newcastle – Ottawa quality assessment scale quality scoring system [Citation15]; two authors scored independently.
We constructed evidence tables detailing study characteristics, outcome measures, MetS definition and study quality. We compared and contrasted studies reporting the connection between MetS and LUTS, summarizing patient characteristics and evidenced results.
Statistical analysis
For the meta-analysis, the pooled mean differences (MDs) for continuous variables and the ln(OR) were used for data pooling.
Meta-analysis was conducted to determine the MD and confidence intervals (CIs) of IPSS total score, IPSS-voiding, IPSS-storage and prostate volume in patients with or without MetS. Ln(OR) were calculated to estimate the risk of having moderate-to-severe LUTS (IPSS ≥ 8) in patients with MetS and to determine the role of each MetS components on this risk among selected studies.
SE[ln(OR)] was calculated through a first-order Taylor series conversion, where SE[ln(OR)] = (1/OR)*SE[OR]. Begg's and Egger's methods were used to assess publication bias. Funnel plots were drawn. Statistical heterogeneity was assessed using the Cochran's Q and I2 statistics. Specifically, statistical heterogeneity was tested using the chi-square test. I2 ≤ 50%, the variation of the studies was considered to be homogenous, the fixed effect model was adopted. If I2 > 50%, there was significant heterogeneity between studies, the random effects model was used. All p values are 2-tailed, α ≤ 0.05 was considered statistically significant (p ≤ 0.05).The analyzes was performed using RevMan software v.5.1 (Cochrane Collaboration, Oxford, UK) and using SPSS v. 19 software (SPSS Inc, IBM Corp, Somers, NY).
Results
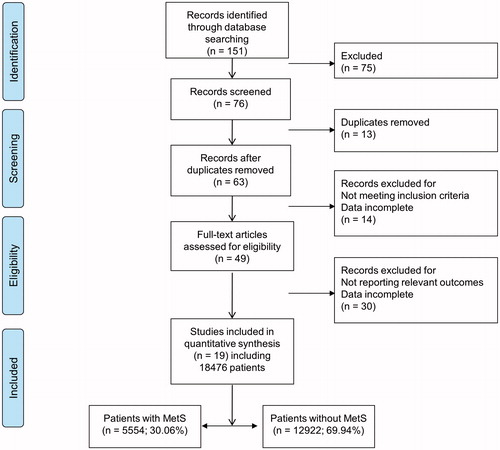
In total, 151 studies were identified from the online databases and relevant references. After evaluating the title and abstract of each study, 75 studies were excluded as they did not meet the inclusion criteria. Subsequently, we carefully read the full texts of the remaining 76 studies and thus 19 studies () were identified as eligible for this systematic review, with a total of 18 476 participants, including 5554 (30.06%) with and 12 922 (69.94%) without MetS (). The quality assessment score was ≥50% in more than half studies (12/19).
Table 1. Studies on the association between MetS components, and LUTS.
International prostate symptom score
International Prostate Symptom Score (IPSS) outcomes in patients with MetS were reported in 14 studies [Citation5,Citation6,Citation8,Citation16–27]. There was statistically significant heterogeneity in these studies (x2 = 40.43, I2 = 68%; p = 0.0001) ().
Figure 2. International Prostate Symptom Score (IPSS) (A), Voiding IPSS (B), Storage IPSS (C) and prostate volume (D) in patients with and without metabolic syndrome (MetS). CI=confidence interval; SD=standard deviation.
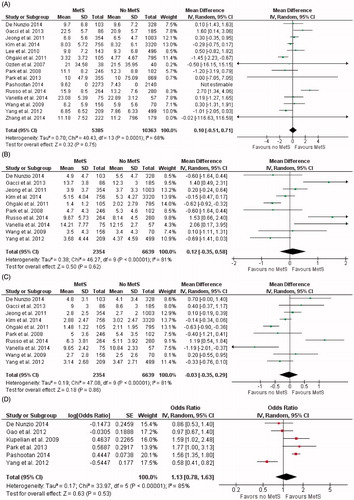
Mean differences of IPSS were not statistical different in men with or without MetS (MD = 0.10; [95% CI −0.51 to 0.71]; p = 0.75).
As concerning IPSS sub scores, pooled analysis demonstrated no statistical differences of IPSS-voiding (MD = 0.12 [95% CI −0.35 to 0.58]; p = 0.62) and IPSS-storage (MD = −0.03 [95% CI –0.35 to 0.29]; p = 0.86) in men with or without MetS ().
Prostate volume
Prostate volume was reported in 12 studies for the pooled meta-analysis [Citation6,Citation8,Citation16–18,Citation20–22,Citation25,Citation26,Citation28,Citation29]. There was statistically significant heterogeneity in these studies (x2 = 67.15, I2 = 84%, p < 0.0001). Patients with MetS exhibited greater prostate volume in patients with MetS with significant statistical differences (MD = 2.18; [95% CI 0.73 to 3.64], p = 0.03) ().
Risk of moderate-to-severe LUTS
A total of six studies examined the association between MetS and the risk of having moderate-to-severe LUTS (IPSS ≥ 8) [Citation16,Citation22,Citation25,Citation30–32]. There was statistically significant heterogeneity in these studies (x2 = 33.97, I2 = 85%, p < 0.000001). In patients with MetS the pooled ORs [95% CI] of having moderate-to-severe LUTS was 1.13 [0.78–1.63]. The test of overall effect was not statistical significant (Z = 0.63, p = 0.53) ().
Figure 3. Risk of having moderate-to-severe lower urinary tract symptoms (IPSS ≥ 8) expressed as odds ratio (OR [95% CI]) in patients with metabolic syndrome (A), in patients with waist circumference ≥90 cm (or ≥ 90 cm for Asians) (B), with HDL < 40 mg/dl (C), with triglycerides ≥ 150 mg/dl (D), in patients with elevated fasting glucose (≥6.1 mmol/L or 110 mg/dL) (E).CI = confidence interval; Homa-index = homeostatic model assessment index.
![Figure 3. Risk of having moderate-to-severe lower urinary tract symptoms (IPSS ≥ 8) expressed as odds ratio (OR [95% CI]) in patients with metabolic syndrome (A), in patients with waist circumference ≥90 cm (or ≥ 90 cm for Asians) (B), with HDL < 40 mg/dl (C), with triglycerides ≥ 150 mg/dl (D), in patients with elevated fasting glucose (≥6.1 mmol/L or 110 mg/dL) (E).CI = confidence interval; Homa-index = homeostatic model assessment index.](/cms/asset/2562133c-93f1-484b-a491-27b2c79158c1/itam_a_1062980_f0003_c.jpg)
MetS components and risk of moderate-to-severe LUTS
A total of 7 studies investigated the role of MetS components on the risk of having moderate-to-severe LUTS [Citation5,Citation21,Citation24,Citation30,Citation31,Citation33].
The impact of waist circumference (WC) was showed in three studies [Citation5,Citation30,Citation31]. There was not a statistically significant heterogeneity in these studies (x2 = 4.42, I2 = 55%, p = 0.11). In patients with WC ≥90 cm for Asian or ≥94 for Caucasian the pooled ORs [95% CI] of having moderate-to-severe LUTS was 1.13 [0.93–1.38]. The test of overall effect was not statistical significant (Z = 1.22, p = 0.22) ().
The role of HDL was obtained in three studies [Citation30,Citation31,Citation33]. There was not a statistically significant heterogeneity in these studies (x2 = 1.15, I2 = 0%, p = 0.56). In patients with HDL <40 mg/dl the pooled ORs [95% CI] of having moderate-to-severe LUTS was 1.22 [0.91–1.64]. The test of overall effect was not statistical significant (Z = 1.32, p = 0.19) ().
The connection between triglycerides and LUTS severity has been demonstrated in four studies [Citation21,Citation30,Citation31,Citation33]. The heterogeneity among these studies was not statistical significant (x2 = 4.57, I2 = 34%, p = 0.21). In patients with triglycerides ≥150 mg/dl the pooled ORs [95% CI] of having moderate-to-severe LUTS was 1.31 [1.01–1.71] with statistical significant (Z = 2.01, p = 0.04) ().
The role of diabetes on LUTS severity has been reported in four studies [Citation21,Citation24,Citation30,Citation31].
There was a statistically significant heterogeneity in these studies (x2 = 12.52, I2 = 76%, p = 0.02). The test of overall effect was not statistical significant (Z = 2.59, p = 0.01) (). In patients with elevated fasting glucose (≥6.1 mmol/L or 110 mg/dL) or previous diagnosis of diabetes, the pooled ORs [95% CI] of having moderate-to-severe LUTS was 1.77 [1.15–2.73]. The test of overall effect was statistical significant (Z = 2.59, p = 0.01) (). The presence of hypertension has been not investigated due to the lack of considerable data.
The relationship between MetS components and IPSS differences were not confirmed in a linear, age, prostate volume and PSA-adjusted, multivariate model, weighing each study for the number of patients enrolled (all p value > 0.1).
Discussion
Connections between lower urinary tract symptoms (LUTS) secondary to benign prostatic enlargement (BPE) and metabolic syndrome (MetS) have been diffusely reported in literature. The underling pathological pathways are mainly attributable to hyperinsulinemia, increased sympathetic nervous system activity and smooth muscle tone of the prostate [Citation27,Citation34].
To this regard, Byun et al. founded that patients with more than one MetS component were significantly more likely to have a larger prostate volume and higher serum PSA level [Citation28].
In a recent meta-analysis on the associations between MetS and BPE, authors found that MetS-induced differences in prostate volumes were almost equally weighted as a factor of age, waist circumference or serum HDL concentration. Hence, obese, dyslipidaemic and aged patients were more at risk of having MetS as a determinant of their increased prostate size. Furthermore, increased-central adiposity, as reflected by waistline, was another MetS-related factor that significantly contributes to variation in prostate enlargement [Citation1].
However, besides previous pathological pathways, the presence of BPE and the observation of MetS should be not directly attributable to the presence of more severe LUTS.
To this regards, literature data have recently underlined some controversies about the connection between LUTS/BPE and MetS. As concerning this, studies from Asian cohorts have evidenced an absent or even negative association between MetS and symptoms severity [Citation17–19,Citation21,Citation30]. Anyway, these studies were conducted in healthy middle-aged men. However, in an age-matched autopsy series, the prevalence and severity of histologic BPH were similar in Caucasian respect Southeast Asian men, despite very different diet and lifestyle [Citation35]. These findings are in contrast with previous observations contradicting the supposition of different geographical impact and MetS at least regarding BPE.
In fact, we would point-out that when analyzing the impact of metabolic disorders on prostatic diseases, it should be considered whom population is affected by. Men above their 60s and with contextual comorbidities seem the most appropriate.
In fact, these discordances may justify results of the current meta-analysis and the lack of statistical significance concerning the mean differences of IPSS, IPSS-storage and IPSS-voiding in patients with and without MetS. Moreover, although only six studies assessed the association between MetS and the risk of having moderate-to-severe LUTS (IPSS ≥ 8), the pooled OR of 1.13 was not statistical significant (p = 0.53).
As concerning prostate volume analysis, herein we have reported significant greater prostate volume in men affected by MetS after the pooled analysis (MD = 2.18; p = 0.03), similarly to recent literature data [Citation1].
The significant increase of prostate volume induced by MetS could be explained by several factors.
In this context, it has been also postulated that MetS could influence LUTS/BPE at an intraprostatic level. Gacci et al. recently reported that MetS was associated not only with an increased prostate volume, but also with a severe intraprostatic inflammation. These observations strengthened the hypothesis that MetS, and hyperinsulinemia-related increase, could boosts a chronic inflammation-driven prostate overgrowth. As concerning MetS components, reduced HDL, and increased triglyceride levels are significantly related to higher prostatic inflammation by secreting interleukin-8 in response not only to oxidized low-density lipoprotein, but also indicating that different MetS features could synergistically boost inflammation and tissue remodeling in LUTS/BPO [Citation8,Citation36].
As concerning central obesity, several studies reported that larger WC (>102 cm) and an increase in BMI were positively correlated with increased LUTS/BPE. In a prospective study on men with no obesity related morbidities as diabetes, impaired fasting glucose, hypertension, or dyslipidaemia, BMI waist circumference were positively correlated with prostate volume [Citation37]. These data have recently been confirmed in the REduction by DUtasteride of prostate Cancer Events (REDUCE) trial [Citation38].
However, results of the present of meta-analysis showed that among MetS components, alteration of waist circumference and serum HDL were not associated with increased OR of having moderate-to-severe LUTS. By contrast, altered serum triglycerides and diabetes were associated with this risk.
These observations could be explained by the increase of insulin-like growth factors (IGFs) and insulin induced by diabetes and the increase of adipokines secondary to hypertriglyceridemia that lead to prostate disarrangement [Citation5,Citation23,Citation39].
The sex hormone milieu may further contribute to the association between MetS and BPE and nowadays, late onset hypogonadism (LOH) is recognized as another MetS-associated condition [Citation40–43].
The hypothesis on the pathogenic mechanism that links metabolic syndrome hypogonadism with LUTS suggests that testosterone may act on the structures of the lower urinary tract because of the presence of androgen receptors on urethral and bladder epithelial cell [Citation44–48]. Also, testosterone and its metabolites have been shown to be able to maintain the reflex activity of the pelvic autonomic nervous system [Citation49].
To this regard, testosterone replacement therapy (TRT) in men with hypogonadism can revert hypogonadal features related to metabolic syndrome but also LUTS (especially voiding disturbance) of patients with LOH. Studies suggest that in addition to improvement in sexual functions, testosterone therapy may also improve LUTS/bladder functions by increasing bladder capacity and compliance and decreasing detrusor pressure at maximal flow in men with LOH.
The theoretical basis to explain the improvement of metabolic syndrome features has been addressed to the improving of obesity parameters (body weight, waist circumference and BMI) and lowering of total cholesterol, LDL cholesterol, triglycerides, fasting blood glucose, HbA1c, and blood pressure. These findings result in a sustained improvement in erectile function and muscle and joint pain, which contributed to an improvement in long-term health-related quality of life [Citation46].
Based on this context, The International Society for the Study of the Aging Male (ISSAM) Hypogonadism panel have recently published Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men [Citation50]. Although recommendations can never replace clinical expertise, it is now strengthened that TRT is able to revert LOH symptoms. Improvement in hypogonadal signs and symptoms occur for different organ systems (body composition, cognitive function, LUTS/BPE, cardiovascular disease, sexual function) that can be translated into an increase of quality of life of men.
However, taking into account all previous considerations, connections between MetS components and more severe LUTS could be also attributable to bladder dysfunction and not to BPE.
In fact, there are also evidences that storage symptoms may be susceptible to different risk factors of MetS, secondary to the possible role of the autonomic nervous system. The activation of the parasympathetic nervous system can cause detrusor muscle contraction and may therefore contribute to detrusor over-activity, which is characteristic of the prevalence of storage symptoms [Citation51].
De Nunzio et al. observed that MetS is associated with an increased risk of storage symptoms in patients with BPE. This subjects presented a higher IPSS storage subscore (p ≤ 0.002). Patients with an IPSS storage subscore ≥4 presented a higher BMI and a higher waist circumference when compared with the less symptomatic patients. Instead MetS was not associated with an increased risk of IPSS and IPSS voiding subscore ≥5 [Citation16], supporting the hypothesis of a contribution of MetS on storage symptoms.
Before concluding some limitations should be addressed. The inclusion of studies with significant heterogeneity and lack of geographical distinction in the analysis could represent potentially bias.
However, some strengths of this study includes the addressing for the first time of the impact of MetS and its components on LUTS/BPE severity and also the inclusion of studies that only reported cohorts with clear MetS definitions.
The association between MetS and LUTS/BPE remain unclear and further observational studies in a population with metabolic disorders should be conducted in order to address their potentially role in determining LUTS/BPE.
Conclusion
Patients affected by MetS and LUTS/BPE are rising and emerging links have been postulated. However, current literature is limited to severe heterogeneity and ethnic disparities and results of the current meta-analysis demonstrated that MetS was not determinant in worsen LUTS/BPE. Moreover, among underling pathways, diabetes and hyper triglyceridemia may represent significant MetS components able to more severe LUTS/BPE. Clinical connections between MetS and LUTS/BPE should be further investigated in order to set new preventive strategies and counteract the development of LUTS/BPE in men at risk.
Declaration of interest
Each author declares no conflict of interest.
References
- Gacci M, Corona G, Vignozzi L, et al. Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis. BJU Int 2015;115:24--31
- Gacci M, Sebastianelli A, Salvi M, et al. Central obesity is predictive of persistent storage LUTS after surgery for benign prostatic enlargement: results of a multicenter prospective study. BJU Int 2015 Jan 18. doi: 10.1111/bju.13038
- Lee RK, Chung D, Chughtai B, et al. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int 2012;110:540–5
- Russo GI, Castelli T, Privitera S, et al. Increase of Framingham cardiovascular disease risk score is associated with severity of lower urinary tract symptoms. BJU Int 2015 Jan 20. doi: 10.1111/bju.13053
- Vanella L, Russo GI, Cimino S, et al. Correlation between lipid profile and heme oxygenase system in patients with benign prostatic hyperplasia. Urology 2014;83:1444 e7–13
- Lee YC, Liu CC, Huang CN, et al. The potential impact of metabolic syndrome on erectile dysfunction in aging Taiwanese males. J Sex Med 2010;7:3127–34
- Vignozzi L, Filippi S, Comeglio P, et al. Tadalafil effect on metabolic syndrome-associated bladder alterations: an experimental study in a rabbit model. J Sex Med 2014;11:1159–72
- Gacci M, Vignozzi L, Sebastianelli A, et al. Metabolic syndrome and lower urinary tract symptoms: the role of inflammation. Prostate Cancer Prostatic Dis 2013;16:101–16
- Vignozzi L, Gacci M, Cellai I, et al. PDE5 inhibitors blunt inflammation in human BPH: a potential mechanism of action for PDE5 inhibitors in LUTS. Prostate 2013;73:1391–402
- Sebastiano C, Vincenzo F, Tommaso C, et al. Dietary patterns and prostatic diseases. Front Biosci (Elite Ed) 2012;4:195–204
- Demir O, Akgul K, Akar Z, et al. Association between severity of lower urinary tract symptoms, erectile dysfunction and metabolic syndrome. Aging Male 2009;12:29–34
- Coyne KS, Sexton CC, Bell JA, et al. The prevalence of lower urinary tract symptoms (LUTS) and overactive bladder (OAB) by racial/ethnic group and age: results from OAB-POLL. Neurourol Urodyn 2013;32:230–7
- Tsai CC, Liu CC, Huang SP, et al. The impact of irritative lower urinary tract symptoms on erectile dysfunction in aging Taiwanese males. Aging Male 2010;13:179–83
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS medicine 2009;6:e1000100
- Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. 26 June 2015
- De Nunzio C, Cindolo L, Gacci M, et al. Metabolic syndrome and lower urinary tract symptoms in patients with benign prostatic enlargement: a possible link to storage symptoms. Urology 2014;84:1181–7
- Jeong JH, Kim ET, Kim DK. Association of metabolic syndrome and benign prostate enlargement in young korean males. Korean J Urol 2011;52:757–62
- Kim JH, Doo SW, Yun JH, Yang WJ. Lower likelihood of having moderate-to-severe lower urinary tract symptoms in middle-aged healthy Korean men with metabolic syndrome. Urology 2014;84:665–9
- Ohgaki K, Hikima N, Horiuchi K, Kondo Y. Association between metabolic syndrome and male lower urinary tract symptoms in Japanese subjects using three sets of criteria for metabolic syndrome and International Prostate Symptom Score. Urology 2011;77:1432–8
- Ozden C, Ozdal OL, Urgancioglu G, et al. The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. European Urol 2007;51:199–203; discussion 4–6
- Park HK, Lee HW, Lee KS, et al. Relationship between lower urinary tract symptoms and metabolic syndrome in a community-based elderly population. Urology 2008;72:556–60
- Park YW, Kim SB, Kwon H, et al. The relationship between lower urinary tract symptoms/benign prostatic hyperplasia and the number of components of metabolic syndrome. Urology 2013;82:674–9
- Russo GI, Cimino S, Fragala E, et al. Insulin resistance is an independent predictor of severe lower urinary tract symptoms and of erectile dysfunction: results from a cross-sectional study. J Sex Med 2014;11:2074–82
- Wang CC, Chancellor MB, Lin JM, et al. Type 2 diabetes but not metabolic syndrome is associated with an increased risk of lower urinary tract symptoms and erectile dysfunction in men aged <45 years. BJU Int 2010;105:1136–40
- Yang TK, Hsieh JT, Chen SC, et al. Metabolic syndrome associated with reduced lower urinary tract symptoms in middle-aged men receiving health checkup. Urology 2012;80:1093–7
- Zhang X, Zeng X, Liu Y, et al. Impact of metabolic syndrome on benign prostatic hyperplasia in elderly Chinese men. Urol Int 2014;93:214–19
- De Nunzio C, Aronson W, Freedland SJ, et al. The correlation between metabolic syndrome and prostatic diseases. European Urol 2012;61:560–70
- Byun HK, Sung YH, Kim W, et al. Relationships between prostate-specific antigen, prostate volume, and components of metabolic syndrome in healthy Korean men. Korean J Urol 2012;53:774–8
- Yim SJ, Cho YS, Joo KJ. Relationship between metabolic syndrome and prostate volume in Korean men under 50 years of age. Korean J Urol 2011;52:390–5
- Gao Y, Wang M, Zhang H, et al. Are metabolic syndrome and its components associated with lower urinary tract symptoms? Results from a Chinese male population survey. Urology 2012;79:194–201
- Kupelian V, McVary KT, Kaplan SA, et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston Area Community Health Survey. J Urol 2009;182:616–24; discussion 24–5
- Pashootan P, Ploussard G, Cocaul A, et al. Association between metabolic syndrome and severity of lower urinary tract symptoms (LUTS): an observational study in a 4666 European men cohort. BJU Int 2014 Sep 17. doi: 10.1111/bju.12931
- Rohrmann S, Smit E, Giovannucci E, Platz EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). Int J Obes (Lond) 2005;29:310–16
- Minutoli L, Altavilla D, Marini H, et al. Inhibitors of apoptosis proteins in experimental benign prostatic hyperplasia: effects of serenoa repens, selenium and lycopene. J Biomed Sci 2014;21:19
- Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of inflammation and benign prostatic hyperplasia on autopsy in Asian and Caucasian men. European Urol 2014;66:619–22
- Vignozzi L, Cellai I, Santi R, et al. Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J Endocrinol 2012;214:31–43
- Lee S, Min HG, Choi SH, et al. Central obesity as a risk factor for prostatic hyperplasia. Obesity (Silver Spring). 2006;14:172–9
- Muller RL, Gerber L, Moreira DM, et al. Obesity is associated with increased prostate growth and attenuated prostate volume reduction by dutasteride. European Urol 2013;63:1115–21
- Russo GI, Cimino S, Fragala E, et al. Relationship between non-alcoholic fatty liver disease and benign prostatic hyperplasia/lower urinary tract symptoms: new insights from an Italian cross-sectional study. World J Urol 2015;33:743--51
- Kaplan SA, Lee JY, O'Neill EA, et al. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013;16:169–72
- Shigehara K, Sugimoto K, Konaka H, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male 2011;14:53–8
- Yassin DJ, El Douaihy Y, Yassin AA, et al. Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year prospective, observational and longitudinal registry study. World J Urol 2014;32:1049–54
- Favilla V, Cimino S, Castelli T, et al. Relationship between lower urinary tract symptoms and serum levels of sex hormones in men with symptomatic benign prostatic hyperplasia. BJU Int 2010;106:1700–3
- Yassin AA, El-Sakka AI, Saad F, Gooren LJ. Lower urinary-tract symptoms and testosterone in elderly men. World J Urol 2008;26:359–64
- Amano T, Imao T, Takemae K, et al. Testosterone replacement therapy by testosterone ointment relieves lower urinary tract symptoms in late onset hypogonadism patients. Aging Male 2010;13:242–6
- Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med 2014;11:1567–76
- Kalinchenko S, Vishnevskiy EL, Koval AN, et al. Beneficial effects of testosterone administration on symptoms of the lower urinary tract in men with late-onset hypogonadism: a pilot study. Aging Male 2008;11:57–61
- Karazindiyanoglu S, Cayan S. The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male 2008;11:146–9
- Chavalmane AK, Comeglio P, Morelli A, et al. Sex steroid receptors in male human bladder: expression and biological function. J Sex Med 2010;7:2698–713
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men Aging Male 2015;18:5–15
- Kirby MG, Wagg A, Cardozo L, et al. Overactive bladder: is there a link to the metabolic syndrome in men? Neurourol Urodyn 2010;29:1360–4