Abstract
Obese men may present hypogonadothrofic hypogonadism, mainly related to higher insulinemia and aromatase activity. Our objectives were to evaluate the relationship of sex-hormones profiles and frequency of depressive symptoms in 43 obese men, in a cross-sectional study. They had 19–60 years, and body mass index 30–50 kg/m2. LH, total and free testosterone (TT and FT), estradiol (E2), sex hormone binding globulin, estradiol/total testosterone ratio (E2/T) were analyzed. Depressive symptoms were evaluated by “beck depression inventory” (BDI), and significant depression was considered if BDI ≥ 16.Thirty-four (80%) presented low TT levels, but only 4 (14%) had low free testosterone and hypogonadism symptoms; 12 of 43 (28%) presented increased E2. Forty five (56%) presented depressive symptoms, but 16 (28% of the 45) had significant depression. BDI correlated positively with E2 (r = 0.407; p = 0.001) and E2/T (r = 0.473; p = 0.001), but not TT or FT. Patients with significant depressive showed higher levels of estradiol (136 ± 48 versus 103 ± 48 pg/ml, p = 0.02) and E2/T (16.0 ± 9.9 versus 9.8 ± 4.6; p = 0.002) (mean ± SD).In conclusion, obese men may present relatively excess of estradiol and deficiency in testosterone, leading to an imbalance between these two hormones. The greater this imbalance, the more depressive symptoms had our patients.
Introduction
The gonadal profile in obese men and women shows unique and paradox patterns, with increasing levels of free estradiol and free testosterone in women, and increasing levels of estradiol, but decreasing levels of testosterone in men [Citation1]. Particularly, obese men have elevated levels of estrone and both free and total estradiol [Citation1]. They also show subnormal levels of free and total testosterone (TT), follicle-stimulating hormone (FSH), luteinizing hormone (LH) and SHBG; all these abnormalities are inversely proportional to the degree of obesity. These alterations in the hypothalamic-pituitary-gonadal axis of obese men complains a moderate state of hypogonadotropic hypogonadism [Citation2–4], which is mainly associated to the amount of total body fat, and not to its distribution [Citation5,Citation6].
The most common explanation for these abnormalities regards to two interrelated mechanisms: the first one is the fat excess “per se”, which is associated with higher aromatase activity with the consequent increases in peripheral conversion of androgens to estrogens; the second mechanism is related to hyperinsulinemia that occurs in insulin resistant obese men. Some authors consider hyperinsulinemia the main responsible factor for both reduced SHBG levels and increased aromatase activity [Citation7,Citation8]. The resultant hyperestrogenism interferes centrally with FSH and LH pulsatilities causing lower testosterone production [Citation9]. In male, GnRH and gonadotropin secretion could be modulated by testosterone and estradiol acting predominantly on hypothalamus or on the pituitary via a feedback regulating mechanisms [Citation9,Citation10].
There is a high comorbidity between depression and obesity. Depressive symptoms have been described in obese men, especially among those with severe obesity, with difficulties in personal relationships, social exclusion, and problems to find a job [Citation11]. It is accepted in a biological model that physical factors of the immunologic neuroendocrine and inflammatory systems make an influence on the relationship of medical disease and depression [Citation12]. There is in fact a 31% increased risk of obese men developing depression overtime, whereas depressive men do not have a significant risk of becoming obese; in women this association is bilateral and stronger [Citation13] Although depressive symptoms in women have been already related to oscillations in estrogen levels at specific periods of reproductive transition (as premenstrual phase of the menstrual cycle, the postpartum period, and perimenopause) some studies showed relations between depressive disorder and estrogen deficiency [Citation14,Citation15].
The association of hypogonadism and depression has been found in non-obese older men with refractory depression: the testosterone replacement therapy associated with antidepressant drugs resulted in better outcomes than in those treated only with placebo and antidepressant drugs [Citation16]. Furthermore, a 12-month prospective study in men with prostate cancer clinically treated with androgen blockade therapy showed an increase in depressive symptoms, which improved significantly during the 18 weeks of follow-up after discontinuation of androgen blockade [Citation17]. Some clinical features of hypogonadism are highly specific (reduced libido, erectile dysfunction, loss of body hair, breast tenderness), tough others are less and may reflects the associated comorbidities (decreased energy, depressive mood, sleep disturbance, increased body fat and body mass index [BMI]).
Considering that both hypogonadism and depression are prevalent in obese men, male hypogonadism is associated with depression. Male hypogonadism in obese men may be due to excess fat, with increased aromatase activity, and/or hyperinsulinemia. The objective of the present study was to examine the association of sexual hormones and depression in obese men. We also evaluated its relations with insulin resistance.
Subjects and methods
This is a cross-sectional study which recruited obese men looking for obesity treatment and attending the Obesity Clinic at Endocrinology Outpatient Clinic of Kidney and Hypertension Hospital, Federal University of São Paulo, and the study protocol has been approved by its Research Ethics Committee (www.cep.unifesp.br). Participants were recruited from subjects fulfilling the inclusion criteria described below, and they have signed the informed consent term previously approved by the Ethical Committee.
Obese male patients with age between 19 and 60 years old, not using illicit drugs, antidepressant, antiandrogenic or antipsychotic medications, anti-obesity drugs or gonadal hormones were included. They were excluded if positive history of delayed puberty, previous bariatric or neurological surgeries, severely acute diseases or emotional traumatic events in the last six months prior to the study, as well with established endocrinopathies, chronic diseases such as hepatopathy, renal insufficiency, psychiatric and neurological diseases. Information regarding life style, sexual life, mental and physical health was gathered, by the same physician, as a semi-structured interview plus the AUDIT-structured questionnaire (alcohol use disorders identification test) [Citation18]. In the first visit, the anamnesis investigated the use of illicit drugs, alcohol, antidepressant, antiandrogenic or antipsychotic medications and associated chronic diseases. In the second visit, a complete physical examination with evaluation of body weight (in kilograms) and height (in meters) was performed. BMI was figured by dividing weight in kilograms by the square of height in meters, and the testicle volumes were measured (cm3) using a Prader orchidometer. The waist circumference was measured at the superior level of iliac crests. Obesity was considered if BMI >30 kg/m2, as defined by the World Health Organization [Citation19].
Evaluation of depressive symptoms
At the third visit, the “Beck Depression Inventory” (BDI) questionnaire was applied to evaluate depressive symptoms [Citation20]. The scale was self-applied, with the supervision of the investigator to discuss doubts. This questionnaire was developed in 1961 by Aaron Beck and provides a self-rating measurement of the behavioral manifestations of depression, and possess a high degree of validity when compared to clinical assessments of American psychiatrists. The questionnaire was translated and adapted for Brazil in 1982 and was validated in 1996 by Gorenstein & Andrade; taking a structural clinical interview done by trained psychiatrists as reference, it has a sensitivity of 70% and a specificity of 87% for depression detection [Citation21,Citation22]. The objective of this questionnaire is to evaluate the intensity of the depressive symptoms using a variety of parameters, such as self-criticism and hopelessness as well as affective, vegetative and cognitive abnormalities. Patients were requested to complete the questionnaire without any intervention. The questionnaire composed of 21 questions and each response was assigned in a score ranging from 0 to 3, with total maximum score of 63. Patients were classified according to the total scores of the BDI as follows: 0–9 are normal, 10–15 with minimal symptoms, 16–19 with mild to moderate depression, 20–29 with moderate to severe depression and 30–63 with severe depression – or if the scores were ≥16, patients were considered as depressive. The high internal consistency of the BDI is reflected as average Cronbach’s alpha coefficient around 0.85 [Citation23].
Laboratory measurements
Also at the third visit, fasting blood samples were collected beginning at 8 a.m., in 10 ml BD vacutainers and taken right away to the Steroid Laboratory of Escola Paulista de Medicina/UNIFESP, under the surveillance of one of the authors (ITNV), immediately centrifuged and frozen to −21 °C until laboratories studies were performed, within 6 months from the blood collection. All determinations were performed in duplicate. Testosterone, LH and FSH levels were determined in three samples collected at 15 min intervals and the mean values of these three determinations were considered for each variable. The levels of estradiol, SHBG and insulin were determined only in the first blood sample. The measurements of total, bioavailable and free testosterone were carried out according to the Endocrine Society Position Statement [Citation24]. TT was measured by radioimmunoassay [Citation25], with local historical controls [Citation26] (limit of detection 0.35 nmol/l, intra-assay coefficient of variation (CV) 7.5 and 13.2% for 16.7 and 3.86 nmol/l, interassay CV 15.5 and 17.6% for 22.87 and 4.97 nmol/l, respectively). The used anti-serum was the anti-testosterone 3-(O-carboximetil)oxima-BSA, the radioactive standard 1,2,6,7 3H-testosterone (250 uCi) (Amersham Biosciences, Uppsala, Sweden). SHBG was measured by immunofluorometric assay (IFMA) [(Delfia Perkin-Elmer, Sao Paulo, Brazil) limit of detection 0.5 nmol/l, intra-assay CV 3.9%, 4.9% and 3.3%; and interassay CV 2.3%, 3.0% and 2.4% for 25.5, 63.9, and 138.0 nmol/l, respectively]. SHBG reference values were 12.9–61.7 nmol/l. Estradiol was measured by the IFMA assay (Delfia Perkin-Elmer, Sao Paulo, Brazil, limit of detection 0.05 nmol/l, intra-assay CV 6.9%, inter-assay CV 9.7%). E2 references values were 60–128 pmol/l; FSH was also measured by IFMA (Delfia Perkin-Elmer, Sao Paulo, Brazil, limit of detection 0.05 nmol/l, intra-assay CV 2.0, 2.8 and 2.2% and interassay CV 1.8, 2.0 and 1.8% for 2.58, 11.5 and 44.8 UI/l, respectively) the reference values were 0.6–9.98 IU/l; and LH was equally measured by IFMA (Delfia Perkin-Elmer, Sao Paulo, Brazil, limit of detection 0.05 nmol/l, intra- assay CV 2.4 and 2.0% for 3.63 and 20.0 UI/l, respectively, and interassay CV 3.1E2%). LH reference values were 1.0–8.4 IU/l. Free testosterone was obtained from serum TT, serum SHBG and serum albumin according to Vermeulen et al. [Citation27] (normal value > 148 pmol/l) The estradiol/TT ratios (E2/T) were calculated using estradiol in pmol/l and TT values in nmol/l. The E2T may be considered an marker of aromatase activity, as this is the enzyme that converts androgens to estrogens [Citation28]. Glycemia were measured by the Enzimatic Colorimetric Method and insulin determinations were also done by IFMA (Auto Delfia, Perkin-Elmer, Sao Paulo, Brazil) with the insulin reference values of 2.34–26.4 µU/ml, and the HOMA-Insulin Resistance were calculated.
Statistical analyses
Data were presented as mean ± SD for numeric variables and by number and percentage for qualitative variables. Comparison among means was performed through Student’s unpaired t-test. Before the t-test, the Kolmogorov–Smirnov test was applied to test for a normally distributed population, with equal variances among the individuals and a 0.05 p value – all the normality test passed in our study. The Contingency Tables were used to determine whether or not the distribution of each group was contingent on the categories it falls in, and the Fisher Exact Test were applied; to a more accurately computed p-value in the significant cases, the Yates Correction Factor was used. Correlations between quantitative variables were tested by Pearson Product Moment Correlation’s correlation. χ2 test was used to test association between variables. For these tests, considering a 0.8 Power and an Alpha error of 0.05 (p-value), the sample size was calculated as 33. Finally, assuming the BDI score as the dependent variable, we ran the Forward Stepwise Multiple Regression Test to evaluate whether independent variables were necessary to predict the dependent the depressive symptoms grade, the Normality Shapiro–Wilk Test and the Constant Variance Test were conducted. The analyses were performed using the software SigmaPlot 13.2 for Windows (Systat Software Inc., San Jose, CA).
Results
We recruited 43 obese men, with a mean of 37.9 years of age (±10.5 of SD), a mean BMI of 38 ± 6 kg/m2, ranging from 30 to 50 kg/m2, and their Beck scores mean was 12.1 ± 6.9, ranging from 1 to 28. None was diabetic.
Analyses on serum TT showed that 34 patients (80%) had values below the lower normal of 13.8 pmol/l, but only 7 (20.5%) reported loss of libido and/or unsatisfactory erection. These seven patients were older than the other 27 with low testosterone levels (46 ± 6.8 versus 36 ± 10.3 years, with p = 0.012), and 6 (14%) were with decreased free testosterone. TT correlated significantly and inversely with the insulin (r −0.30; p = 0.05) and HOMA-IR levels (r −0.37; p = 0.015), reflecting the relationship between androgens and insulin sensitivity. These data are shown in the matrix of correlation ().
Table 1. Correlation matrix – hormones versus insulin resistance aspects.
Twelve out of the 43 patients (28%) presented serum estradiol levels above 128 pmol/l, characterizing hyperestrogenism. No correlations were found between estradiol and both total and free testosterone levels or between estradiol and LH levels. Analysis of the scores obtained with the BCI revealed that 24 out of 43 patients (56%) presented depressive symptoms, and 12 (28%) presented significant depression. The score values did not correlate with BMI or age (power of tests < 0.80). Although significant correlation was not observed between both total and free testosterone levels and the scores obtained from BDI, a significant association was observed between the occurrence significant depression and TT levels below 13.8 pmol/l (p = 0.0001).
When we compared patients with no or mild symptoms of depression (BDI scores < 16) with the patients with significant depression, no differences in metabolic parameters were found. However, those with BDI ≥ 16 showed clearly higher levels of estradiol (136 ± 48 versus 103 ± 48 pg/ml, p = 0.02) and of the E2/T (16.0 ± 9.9 versus 9.8 ± 4.6, respectively; p = 0.002). These data are presented in . Also, the proportion of patients with estrogen excess was greater in the significant depressive group (16% in the no to mild symptoms group versus 50% in the significantly depressive group; p < 0.05).
Table 2. Comparison of clinical and laboratorial characteristics in patients with no to mild depressive symptoms (BDI scores <16) versus the most depressive patients (moderate to severe BDI scores ≥16).
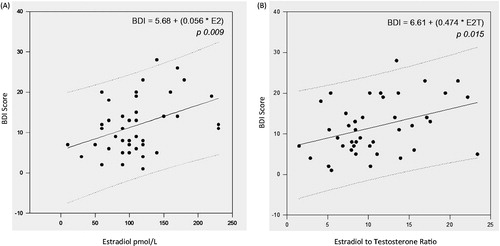
In contrast, serum estradiol levels showed a positive and significant correlation with BDI scores (r = 0.399; p = 0.009). The E2/T values, that in theory would reflect aromatase activity, also showed a significant correlation to Beck scores (r = 0.37; p = 0.015). A Standard Multiple Linear Regression Test could not be done with both estradiol and the ratio between estradiol and E2/T, because of the multicolinearity between these valuables. So, we did manually two models, one with estradiol and other with E2/T, both with BDI score as dependent valuable, and controlling for BMI and age. These results confirmed that the E2/T ratio significantly and positively increases the scores of BDI, or the grade of depression (R = 0.38; R2 = 0.15 and p = 0.015). Estradiol was also significantly interfering in the BDI score (R = 0.42, R2 = 0.17; p = 0.009), Testosterone, total or free, was rejected in this model with a power of 0.91. The data are represented in .
Discussion
In our study, we found that 80% of individuals presented low TT levels, but only 14% presented with decreased free testosterone levels; these were almost all the patients with loss of libido. However, testosterone levels did not correlate with the BDI Score, contrasting with estradiol levels and, most importantly with the E2/T values. We found that 28% of patients presented hyperestrogenism, and estradiol and E2/T values were higher in the most depressive patients.
In males, the major source of circulating estrogens is the aromatization of androgens as a consequence of the action of the enzymatic complex known as aromatase. This is widely expressed in a number of male tissues, including the testis and brain, and mainly the adipose tissue [Citation29]. As shown by Finkenstein et al. [Citation30], estrogens elicit a variety of physiological responses in men and may contribute to modulation of sexual function. In addition, many important actions of testosterone on body fat, bone turnover, bone mass density and HDL cholesterol are mediated predominantly or in part by conversion of testosterone to estradiol and subsequent estrogen action [Citation31].
The estradiol levels did not correlate either with insulin levels or inversely with testosterone levels in our patients, suggesting that mechanisms other than obesity and insulin resistance might also be affecting its levels. When analyzing the aromatase polymorphism, Hammoud et al. showed that the interaction of weight and estradiol levels in men varies according to this enzyme polymorphism. It was found that the effect of increasing degrees of obesity on estradiol levels, as well as the impact of weight loss on these levels, were dependent on the number of TTTAn repeats in the enzyme polymorphism [Citation32].
We observed in our patients a significant and inverse relation between TT and insulin levels, which could be attributed to obesity since hyperinsulinemia, as a result of insulin resistance, is proportional to the amount of adipose tissue. Thus, the more amount of adipose tissue the higher the insulin levels, the higher the conversion from testosterone to estradiol and the lower the testosterone levels. The insulin resistance itself has been shown to contribute to hypogonadism as it is related to decreases in Leydig cells function, reducing the testosterone production [Citation33]. On the other hand, low testosterone levels would predispose to body fat centralization, which in turn would result in increases in insulin resistance and hyperinsulinemia. This could be contributing to the negative correlation observed between testosterone and insulin serum levels. In fact, it has been already demonstrated that individuals with primary hypogonadism compared with controls showed a more centralized body fat distribution and higher insulin levels [Citation9,Citation34]. In addition, it was shown that there was a significant decrease in insulin resistance in hypogonadal men after 6 months of testosterone replacement therapy [Citation35].
Considering the psychiatric illness in obese men, it is not more prevalent than in non-obese individuals, except for depressive disorders [Citation36–38]. In our study we observed a frequency of 60% of depressive symptoms, and the depressive scores determined were not correlated with BMI. Thus, we have presumed that other factors besides obesity would be involved in the development of the depressive process.
Most of the studies that correlated depression, depressive state and depressive symptoms to low testosterone levels were performed in elderly with controversial results. In a cohort study with men up 70 years old, mentally and physically healthy, there was no association between low testosterone levels (total and free) and the severity of the depressive symptoms [Citation39]. In the “Rancho Bernardo Study”, which used BDI in 856 men with ages ranging from 50 to 89 years, an inverse correlation was observed between free testosterone levels and depression scores [Citation40]. Also, in a study conducted by Delhez et al. [Citation41], with 754 men ages 50–70 years diagnosed with andropause, a negative correlation was observed between the severity of depression, as assessed by the Carroll Rating Scale, and the free testosterone levels [Citation41]. However, subjects with high score on this scale did not exhibit different free testosterone levels compared with subjects with smaller depressive score. Yet, in another study, the antidepressant efficacy of testosterone replacement in depressed, hypogonadal men showed no better results than the placebo [Citation42]. In summary, though it is a generally admitted that testosterone has ansiolytic and antidepressant effects in women, men and animals [Citation43], data are not consistent concerning the association between low testosterone levels and depressive symptoms. In our study we observed an association between more severe depressive symptoms and low testosterone levels, with no significant correlation between these parameters.
By the time our study was completed, no reports were found showing an association of higher levels of estradiol and mood abnormalities in obese men. On the contrary, many authors defend that higher estradiol levels in men do not have any pathologic meaning [Citation35,Citation18,Citation19]. We found that the most depressive patients, with moderate to severe depressive symptoms, had significantly higher levels of estradiol and E2/T values than patients without or with mild symptoms of depression. There was also a positive correlation between Beck scores with estradiol and with E2/T. In women, the incidence of depressive symptoms is related to reproductive stages associated with fluctuations in estradiol production [Citation13]. Yet, the substrates for brain aromatization can arise from peripheral or central steroidogenesis [Citation44]. Similarly, in obese men, an increased aromatase activity could also contribute to fluctuations in the amount of estrogen in the brain which, acting in the mood related neuronal centers would cause depressive symptoms in functionally relevant concentrations. A study in 2005 showed a small association between higher estradiol and free estradiol with depression in a geriatric population [Citation45]; this population, as the obese, may also have an imbalance between estradiol and testosterone [Citation46]. More recently, a study highlighted the association of longer CAG repeat polymorphism of the androgen receptor with alterations in psychic symptoms (mainly anxiety) and its relationship with estradiol [Citation47].
The International Society for the Study of the Aging Male Hypogonadism panel presented an update of its recommendations in 2015. They suggest measurement of serum testosterone level in all men with obesity and diabetes mellitus type 2 (Level 2b, Grade A). They also suggest that testosterone replacement therapy may be safely utilized to ameliorate somatic and psychological frailty symptoms in association with improved anthropometric and glycometabolic parameters in aging, overweight men with late onset hypogonadism and impaired fasting glucose [Citation48]. However, in obese male, testosterone replacement therapy could increase their estradiol levels. As a secondary form of hypogonadism, and its consequent hormonal imbalance discussed in this article, alternative treatments should be offered, e.g. the use of antiestrogens [Citation49]. In a recent discussion in “Clinical Decisions case” presented in a forum at the NEJM [Citation50], the compilation of positions in the medical community worldwide showed that 57%, including a majority of the readers in 54 countries, recommended against starting testosterone therapy [Citation51]. In fact, more studies are necessary regarding treatment of hypogonadism in the obese male population.
In conclusion, our results indicate that sub clinical hypogonadal states in obese men are associated to a relative excess of estradiol and deficiency in testosterone production, reflecting an imbalance between these two hormones. The more this balance is compromised, the more depressive symptoms have our patients. It is possible that the differences in aromatase activity lead to the different estrogen levels that were seen in our patients. Studies in which estradiol production or action could be inhibited would help to clarify the role of estradiol levels in the genesis of depressive symptoms in male obesity.
Acknowledgements
We thank Ms. Gianni Mara Silva dos Santos, from the Applied Statistics Sector of UNIFESP for the special help in statistics.
Declaration of interest
This work was supported by the Brazilian “National Council for ScientificResearch” (CNPq).
References
- Zumoff B. Hormonal abnormalities in obesity. Acta Med Scand Suppl 1988;723:153–60
- Strain GW, Zumoff B, Kream J, et al. Mild hypogonadotropic hypogonadism in obese men. Metabolism 1982;31:871–5
- Pasquali R, Casimirri F, Cantobelli S, et al. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism 1991;40:101–4
- Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clinic Endocrinol Metab 1993;76:1140–6
- Strain G, Zumoff B, Rosner W, Pi-Sunyer X. The relationship between serum levels of insulin and sex hormone-binding globulin in men: the effect of weight loss. J Clin Endocrinol Metab 1994;79:1173–6
- Lima N, Cavaliere H, Knobel M, et al. Decreased androgen levels in massively obese men may be associated with impaired function of the gonadostat. Int J Obes Relat Metab Disord 2000;24:1433–7
- Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Endocrinol. Metab 1994;79:997–1000
- Zumoff B, Miller LK, Strain GW. Reversal of the hypogonatropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism 2003;52:1126–8
- Chimento A, Sirianni R, Casaburi I, Pezzi V. Role of Estrogen Receptors and G Protein-Coupled Estrogen Receptor in Regulation of Hypothalamus-Pituitary-Testis Axis and Spermatogenesis. Front Endocrinol (Lausanne) 2014;5:1–8
- Tilbrook AJ, Clarke IJ. Negative feedback regulation of the secretion and actions of gonadotropin-releasing hormone in males. Biol Reprod 2001;64:735–42
- Seidman SN, Araujo AB, Roose SP, Mckinlay JB. Testosterone level, androgen receptor polymorphism, and depressive symptoms in middle-aged men. Biol Psychiatry 2001;50:371–6
- Kloetron VPH, Osj V. Stress, the brain and depression. Cambridge: University Press. Cambridge; 2004
- Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010;67:220–9
- Borrow AP, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: Implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 2014;54:13–25
- Douma SL, Husband C, O’Donnell ME, Barwin BN. Estrogen related mood disorders: reproductive life cycle factors. ANS Adv Nurs Sci 2005;28:364–75
- Shores MM, Sloan KL, Matsumoto AM, et al. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004;61:162–7
- Almeida OP, Waterreus A, Spry N, et al. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology 2004;29:1071–81
- Saunders JB, Aasland OG, Babor TF, et al. Development of the alcohol use disorders identification test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction 1993;88:791–804
- World Health Organization WHO Technical Report: Diet, Nutrition and the Prevention of Chronic Disease. Available from: http://www.who.int [last accessed 2 May 2015]
- Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–71
- Gorenstein C, Andrade L. Validation of a Portuguese version of the Beck Depression Inventory and the State–Trait Anxiety Inventory in Brazilian subjects. Braz J Med Biol Res 1996;29:453–7
- Gomes-Oliveira MH, Gorenstein C, Lotufo-Neto F, et al. Validation of the brasilian portuguese version of the Depresion Beck Inventory in a community sample. Braz J Med Biol Res 1996;29:453–7
- Beck AT, Steer RA, Brown GK. BDI-II: Beck Depression Inventory Manual. 2nd ed. San Antonio: Psychological Corporation; 1996
- Rosner W, Auchus J, Azziz R, et al. Position statement utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society Position Statement. J Clin Endocrinol Metab 2007;92:405–13
- Lox CD, Christian CD, Heine MW. A simple radioimmunoassay for testosterone. Am J Obstet Gynecol 1974;118:114–18
- Callou de Sá EQ, Feijó de Sá FC, e Silva RS, et al. Endogenous oestradiol but not testosterone is related to coronary artery disease in men. Clin Endocrinol 2011;75:177–83
- Vermeullen A, Verdonck L, Kaufman JM. A clnical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72
- Jiang J, Tang NL, Ohlsson C, et al. Association of genetic variations in aromatase gene with serum estrogen and estrogen/testosterone ratio in Chinese elderly men. Clin Chim Acta 2010;411:53–8
- Simpson ER, Mahendroo MS, Means GD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev 1004;15:342–55
- Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 2013;369:1011–22
- Matsumoto AM. Reproductive endocrinology: estrogens – not just female hormones. Nat Rev Endocrinol 2013;9:693–4
- Hammoud A, Carrell DT, Meikle AW, et al. An aromatase polymorphism modulates the relationship between weight and estradiol levels in obese men. Fertil Steril 2010;9:1734–8
- Pitteloud N, Hardin M, Dwyer AA, et al. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab 2005;90:2636–41
- Pagotto U, Gambineri A, Pelusi C, et al. Testosterone replacement therapy restores normal Ghrelin in hypogonadal men. J Clin Endocrinol Metab 2003;88:4139–43
- Bhasin S, Cunningham GR, Hayes FJ, et al. Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536–59
- Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010;67:220–9
- Licinio J, Wong M-L. The interface of obesity and depression: risk factors for the metabolic syndrome. Rev Bras Psiquiatria 2003;25:196–7
- Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ. Are the obese at greater risk for depression? Am J of Epidemiol 2000;152:163–70
- T’Sjoen GG, De Vos S, Goemaere S, et al. Sex steroid level, androgen receptor polymorphism, and depressive symptoms in healthy elderly men. J Am Geriatr Soc 2005;53:636–42
- Barrett-Connor E, Von Mühlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab 1999;84:573–7
- Delhez M, Hansenne M, Legros J-J. Andropause and psychopathology: minor symptoms rather than pathological ones. Psychoneuroendocrinol 2003;28:863–74
- Seidman SN, Spatz E, Rizzo C, Roose SP. Testosterone replacement therapy for hypogonadal men with major depressive disorder: a randomized placebo-controlled clinical trial. J Clin Psychiatry 2001;62:406–12
- McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol 2014;35:42–57
- Schlinger BA, Remage-Healy L, Rensel M. Establishing regional specificity of neuroestrogen action. Gen Comp Endocrinol. 2014;205:235–41
- Schneider G, Zitzman M, Gromoll J, et al. The relation between sex hormone levels, the androgen receptor CAGn-polymorphism and depression and mortality in older men in a community study. Psychoneuroendocrinol 2013; 38:2083–90
- Srilatha B, Adaikan PG. Endocrine milieu and erectile dysfunction: is oestradioltestosterone ratio imbalance, a risk factor in the elderly? Asian J Androl 2011;13:569–73
- Schneider G, Nienhaus K, Gromoll J, et al. Sex hormone levels, genetic androgen receptor polymorphism, and anxiety in ≥50-year-old males. J Sex Med 2011;8:3452–64
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015;18:5–15
- Corona G, Rastrelli G, Maggi M. The pharmacotherapy of male hypogonadism besides androgens. Expert Opin Pharmacother 2015;16:369–87
- Swerdloff R, Anawalt BD. Clinical decisions. Testosterone-replacement therapy. N Engl J Med 2014;371:2032–4
- Campion EW. Executive Editor Annalsis of pooling results. Available from: http://www.nejm.org/doi/full/10.1056/NEJMclde1406595 [last accessed Jul 2015]