Abstract
Whether testosterone replacement therapy (TRT) is a lifelong treatment for men with hypogonadism remains unknown. We investigated long-term TRT and TRT withdrawal on obesity and prostate-related parameters. Two hundred and sixty-two hypogonadal patients (mean age 59.5) received testosterone undecanoate in 12-week intervals for a maximum of 11 years. One hundred and forty-seven men had TRT interrupted for a mean of 16.9 months and resumed thereafter (Group A). The remaining 115 patients were treated continuously (Group B). Prostate volume, prostate-specific antigen (PSA), residual voiding volume, bladder wall thickness, C-reactive protein (CRP), aging male symptoms (AMS), International Index of erectile function – erectile function (IIEF-EF) and International Prostate Symptoms Scores (IPSS) were measured over the study period with anthropometric parameters of obesity, including weight, body mass index (BMI) and waist circumference. Prior to interruption, TRT resulted in improvements in residual voiding volume, bladder wall thickness, CRP, AMS, IIEF-EF, IPSS and obesity parameters while PSA and prostate volume increased. TRT interruption reduced total testosterone to hypogonadal levels in Group A and resulted in worsening of obesity parameters, AMS, IPSS, residual voiding volume and bladder wall thickness, IIEF-EF and PSA while CRP and prostate volume were unchanged until treatment resumed whereby these effects were reversed. TRT interruption results in worsening of symptoms. Hypogonadism may require lifelong TRT.
Introduction
As men age testosterone levels decline, a feature that may be enhanced by and lead to specific concurrent diseases such as obesity, metabolic syndrome (MetS), type 2 diabetes mellitus, erectile dysfunction (ED) and lower urinary tract symptoms (LUTS) [Citation1]. Men with late-onset hypogonadism (LOH), defined as “a clinical and biochemical syndrome associated with advancing age and characterized by symptoms and a deficiency in serum testosterone levels (below the young healthy adult male reference range)” [Citation2], experience decreased quality of life (QoL) [Citation3]. Due to the beneficial effects of testosterone on several of the signs and symptoms of androgen deficiency in aging men and on QoL, testosterone replacement therapy (TRT) is more frequently being considered as a beneficial adjunctive treatment in such individuals [Citation3]. However, studies are still required to assess the long-term safety of TRT and whether TRT is a required and beneficial lifelong therapy.
LUTS are often regarded as a hallmark of benign prostatic hyperplasia with an increased incidence as men age [Citation4]. Many men with LOH experience LUTS which can cause a considerable amount of distress leading to reduced QoL. Hypogonadism has been reported in approximately 20% of elderly men with LUTS [Citation5]. Few studies have investigated the effects of TRT in testosterone-deficient men on parameters and symptoms of LUTS. We reported, in a 5-year prospective, observational, longitudinal, registry study, that TRT was associated with improvements in LUTS, even after correction for the use of PDE5i to treat ED [Citation6]. Patients also exhibited a reduction in weight, waist circumference and body mass index (BMI) at the end of the study period. LUTS is associated with several features of MetS, including obesity in epidemiological studies [Citation7–9] and prostatic diseases are closely associated with obesity [Citation10]. Furthermore, an inverse relationship between testosterone levels and obesity is apparent within the literature and clinical studies have demonstrated that TRT can improve anthropometric measures of obesity, body composition and adiposity [Citation11]. Whilst the mechanisms underlying this potential interrelationship between testosterone, obesity and LUTS is not known currently, evidence from some studies indicate that TRT may be a useful therapy for improving metabolic and urinary symptoms as well as comorbidities of LOH [Citation6,Citation11].
To date, few studies have investigated the effects of TRT withdrawal in androgen-deficient patients and conflicting data exist in the literature. Early investigations by Schroeder et al. demonstrated that while the effects of TRT on bone mineral density and muscle mass returned to baseline, reduction in central and peripheral fat were largely maintained after 3-months treatment withdrawal in overweight older men with low testosterone [Citation12,Citation13]. In a small retrospective intervention study, TRT withdrawal was also shown not to increase proinflammatory cytokine production by antigen-presenting cells in men with T2D and partial androgen deficiency over 3-months [Citation14]. More recently, 24 severely obese hypogonadal men who demonstrated significant improvements in measures of cardiovascular disease and risk factors during 54-weeks of reduced-calorie-diet, physical exercise and TRT saw a return to baseline in cardiovascular and metabolic parameters over a subsequent 24-week withdrawal of TRT [Citation15]. Improvements in some metabolic parameters, including fat mass and blood pressure did, however, remain during withdrawal, although this period was only of relatively short duration. It is considered that positive effects of testosterone treatment on weight and body composition may take between 6 and 12 months, particularly in morbidly obese men [Citation11]. Comparatively, these benefits may be maintained over the short term but may gradually be lost over longer durations. In this retrospective registry study, we aimed to analyze the effect of TRT withdrawal (mean withdrawal 16.9 months) on parameters of obesity and LUTS in hypogonadal men previously treated with TRT (mean treatment duration 57.1 months), and the effect of subsequent treatment reinstatement (mean treatment period 14.5 months).
Methods
This was a population-based prospective, cumulative, registry study. A total of 262 hypogonadal men diagnosed with a total testosterone concentration of ≤3.5 ng/ml and IIEF-5 scores <21 (reference range adopted from [Citation16]) presenting with ED (mean age 59.49 ± 8.72 years). Approval from the ethics committee in line with guidelines formulated by the German Ärztekammer (German Medical Association) was obtained. Patients were enrolled following signed an informed written consent.
Patients received long-acting parenteral testosterone undecanoate 1000 mg (Nebido®, Bayer Pharma, Berlin, Germany) in 12-week intervals for a maximum of 126 months (10.5 years). In 147 men (Group A), testosterone therapy after long-term treatment for a mean duration of 65.5 months was interrupted for a mean duration of 16.9 months due to reimbursement problems (n = 140) or a diagnosis of prostate cancer (n = 7), and resumed thereafter for a mean duration of 14.5 months. The remaining 115 patients (Group B) were treated without interruption. To compare on-treatment to off-treatment periods, three periods of equal duration were defined, as pre (65.5 months), during (16.9 months) and post (14.5 months) interruption, where “post” indicates the period following resumption of TRT. For comparison, the same periods were analyzed for patients who continued TRT throughout. Subjects who dropped out of the study or are currently under observation were included in the continuous treatment group up to the point where TRT was ceased.
Prostate volume, residual voiding volume and bladder wall thickness were routinely measured by ultrasonography at every other visit. International Prostate Symptom Scores (IPSS), aging male symptoms (AMS), international index of erectile function – erectile function (IIEF-EF) questionnaires were assessed at each treatment visit and blood samples taken to measure prostate-specific antigen (PSA), serum testosterone concentrations and C-reactive protein (CRP). Anthropometric parameters of obesity, including weight, waist circumference and BMI were recorded throughout the study period.
Statistical analyses were performed with SPSS® version 18 (Chicago, IL). Data are expressed as mean group values with standard deviations at each time point of preinterruption, during interruption and post-treatment periods. For subjects who received continuous treatment, equivalent time periods were calculated in reverse order from their last visit. Baseline and outcome data were compared between groups using t-tests. Clinical parameters were compared between groups across the treatment periods using mixed-effects, repeated-measures model with period, group and their interaction as fixed effects. Analysis of variance was used to compare categorical and continuous variables. Comparisons between categorical variables were assessed using χ2 tests. A value of p < 0.05 was considered significant.
Results
A total of 262 registry participants with a mean age of 59.49 ± 8.72 years (minimum 19, maximum 84 years) contributed 2088.5 patient-years (25 062 patient-months). Total testosterone concentrations in Group A were 16.54 nmol/l preinterruption, this dropped significantly to 7.48 nmol/l during (p < 0.0001) and increased again to 18.5 nmol/l post TRT interruption (p < 0.0001). In Group B, testosterone remained stable at 19.61, 19.76 and 19.65 nmol/l respectively (p = ns).
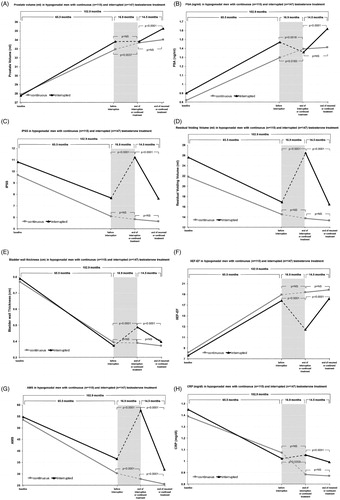
Prostate volume was significantly increased with treatment in both groups. In Group A, prostate volume did not increase further from preinterruption 33.8 ml to during interruption of TRT 33.8 ml. Post-interruption prostate volume increased to 35.3 ml (p < 0.0001). In Group B, prostate volume increased slightly but significantly from 33 to 33.7 ml (p = 0.0037) pre- to during-interruption and further increased to 34 ml post interruption period although not significantly so. PSA in Group A was 1.9 ng/ml preinterruption and decreased to 1.4 ng/ml during-interruption. Post-interruption PSA increased again to 1.6 ng/ml (p < 0.0001). In Group B, PSA remained stable at 1.3, 1.4 and 1.4 ng/ml. IPSS decreased with treatment in both groups. In Group A, IPSS increased from 7.7 preinterruption to 11.2 (p < 0.0001) during, then decreased again to 7.6 post-TRT interruption (p < 0.0001). In Group B, IPSS decreased slightly from 6.1 to 5.8 and 5.7 with continuous treatment. Preinterruption residual voiding volume in Group A increased from 16.9 to 26.5 during-interruption (p < 0.0001) and decreased again to 16.5 ml post interruption (p < 0.0001). In Group B, residual volume decreased slightly from 14.5 to 13.7 ml from pre- to during-interruption (p < 0.0001) and further decreased to 13.3 ml post-interruption (p < 0.0001). Bladder wall thickness decreased greatly in both groups with TRT to preinterruption. In Group A, bladder wall thickness significantly increased from preinterruption to during-interruption 0.37–0.49 cm (p < 0.0001), then decreased again at post-interruption to 0.39 cm (p < 0.0001). Group B bladder wall thickness did not significantly decrease from preinterruption (0.39 cm) to during-interruption (0.39 cm) to post-interruption (0.37 cm).
IIEF-EF increased following TRT in both groups over a mean of 65.5 months prior to interruption from 8.2 to 18.9 and 7.6 to 17.8 in Groups A and B, respectively. Following interruption IIEF-EF scores in Group A significantly decreased to 12.5 (p < 0.0001), whereas Group B had no significant change (19.4) throughout equivalent interruption period. Reinstatement of TRT significantly increased IIEF-EF to 18.19 (p < 0.0001) in Group A, while no significant change was observed in Group B where scores slightly increased to 19.9. AMS scores fell from 54.8 to 36.5 and 53.5 to 30.5 with TRT in Groups A and B, respectively. Interruption significantly increased AMS score to 57.7 (p < 0.0001) in Group A compared to before interruption, whereas Group B saw AMS score continue to drop significantly to 27.8 (p < 0.0001). Following a mean of 14.5 months TRT reinstatement, AMS score was significantly reduced to 31.9 in Group A (p < 0.0001). AMS scores in Group B continued to significantly decrease to 25.5 (p < 0.0001).
TRT reduced CRP levels in both Groups A and B from 1.45 to 1.02 and 1.39 to 1.07 respectively. Interruption of TRT in Group A led to a slight but non-significant increase to 1.05. In contrast, Group B saw CRP levels continue to decline significantly to 0.88 (p = 0.0359). No further decline was observed in Group B following 14.5 months continued treatment, whereas TRT reinstatement reduced CRP levels in Group A to 0.99 (p = 0.0001).
Waist circumference was greatly reduced following TRT in both groups. In Group A, waist circumference increased from 100.2 cm preinterruption to 105.4 cm during-interruption (p < 0.0001) and decreased again to 102.3 cm post-interruption (p < 0.0001), similar to preinterruption measures. In Group B, waist circumference continuously declined significantly from 98.4 cm pre- to 97.2 cm during interruption and 95.7 cm post-interruption (p < 0.0001). Weight also decreased in both groups with TRT. In Group A, mean weight was 92.1 kg preinterruption and increased to 97.1 kg during (p < 0.0001) then decreased again to 94.4 kg post-interruption (p < 0.0001). In Group B, weight continuously significantly declined from 87.7 kg preinterruption to 86.2 kg during-interruption (p < 0.0001) and to 84.4 kg post-interruption (p < 0.0001). BMI decreased in both groups from baseline to preinterruption. In Group A, BMI increased from 29.2 kg/m2 preinterruption to 30.7 kg/m2 during interruption (p < 0.0001) then decreased again to 29.9 kg/m2 post-interruption (p < 0.0001). BMI continued to significantly decrease in Group B from preinterruption 27.7 kg/m2 to during interruption 27.3 kg/m2 (p < 0.0001) and to 26.7 kg/m2 post-interruption (p < 0.0001).
During TRT withdrawal, four patients suffered acute urinary retention and two underwent transurethral resection of the prostate (TURP). One patient underwent TURP due to progressive severe obstructive symptoms.
Discussion
Hypogonadism is now a recognized entity with clinically associated features and comorbidities including obesity, MetS, T2D, ED and LUTS. Treatment of hypogonadism with TRT, although still widely debated, has been demonstrated in the majority of studies to improve parameters of obesity and body composition, erectile function, symptoms of diabetes and QoL which has in turn contributed to the rapid rise in TRT use over the past decade [Citation11]. Despite this increased use of testosterone as a therapy for hypogonadism and testosterone deficiency, relatively little is known about the long-term and even lifelong treatment strategy needed with respect to safety and maintenance of symptom benefits. In this study, we have demonstrated that long-term TRT improves parameters of obesity and LUTS without prostate safety concerns in hypogonadal men and the new finding that a mean 16.9-month treatment interruption abrogates these effects yet treatment reinstatement subsequently restores improvements.
Relatively little is known about the required duration of treatment with TRT in hypogonadism. Many studies demonstrating beneficial effects of TRT are short-term studies. Some long-term observational follow-up studies report substantial and continuous improvements in weight and waist circumference and decreased BMI in hypogonadal men treated with testosterone undecanoate over a 5-year period [Citation17–22]. Similarly, we and others have shown that long-term 5-year TRT is associated with improvements in LUTS [Citation6,Citation15]. Almost all TRT studies, however, are not specifically designed to assess the time course of treatment effects thus limiting the ability to interpret results. This highlights the need for long-term investigations of sustainability of clinical improvements with and without treatment withdrawal. Indeed, the differential responses to continuous TRT over time are of importance both for the management of patient expectations and their adherence to therapy as well as for future TRT clinical trial design for the production of meaningful and quantifiable measurements.
To date, few studies have investigated the consequence of withdrawing TRT in androgen-deficient patients after substantial treatment duration which has brought about beneficial effects. Of these limited studies, conflicting data exist. Schroeder et al. demonstrated that 12 weeks treatment with the anabolic steroid oxandrolone resulted in significant reductions in total, trunk and appendicular fat assessed by dual energy X-ray absorptiometry scan in overweight older men with low testosterone [Citation13]. These changes in body composition were accompanied by improvements in estimates of insulin sensitivity and alterations in lipid profiles. Twelve weeks after discontinuing oxandrolone treatment, approximately 83% of the observed reductions in total, trunk and extremity fat were maintained suggesting that androgen therapy produced significant and sustainable improvements in body composition that were associated with improvements in measures of insulin sensitivity [Citation13]. In agreement, Francomano et al. demonstrated that the improvements in obesity parameters (BMI, WC, subtotal and trunk fat) seen in severely obese hypogonadal men after 54-weeks of reduced calorie diet, physical exercise and TRT versus diet and exercise intervention alone were maintained 24-weeks post-treatment withdrawal [Citation15]. However, improvements in weight, lean mass and cardiovascular risk score, evaluated using the risk engine derived from the “Progetto Cuore study” [Citation15], returned to baseline. Sustained anti-inflammatory effects of TRT after treatment cessation were also demonstrated in men with T2D and partial androgen deficiency [Citation14]. The reduction or complete abrogation of spontaneous ex vivo production of proinflammatory cytokines (interleukin-1β, interleukin-6 and tumor necrosis factor-α) by circulating monocytes and dendritic cells observed following 12 months of intramuscular testosterone enanthate was maintained 3 months following treatment withdrawal. These studies only monitored parameters over a relatively short duration of testosterone withdrawal, particularly when it is considered that effects of testosterone on weight and body composition may take between 6 and 12 months [Citation11]. Therefore, the maintenance of some measures in these studies may occur over the short-term but may be gradually lost over longer durations. Indeed, we present evidence in the current study that a period of 16.9 months TRT withdrawal leads to loss of treatment improvements in obesity, LUTS, ED and QoL parameters and have previously reported similar effects on metabolic parameters [Citation23].
Testosterone is associated with QoL and ED and although it has been difficult to relate plasma testosterone to LUTS preliminary evidence also indicates a relationship between the two [Citation24]. LUTS is an important determinant of QoL. As previously reported [Citation6,Citation25], TRT was associated with improvements in QoL, ED and LUTS in the present study. Furthermore, inflammation is implicated in ED, voiding function and depression, therefore, QoL. Giltay et al. [Citation26] have previously demonstrated that CRP, a marker of systemic low-grade inflammation, is increased in hypogonadal men and that TRT reduces this. In parallel, an association between CRP and AMS was observed further linking inflammation, QoL and ED with testosterone. Previous investigations, however, did not assess TRT withdrawal and the subsequent effects of treatment reinstatement post-withdrawal which is shown to re-establish improvements in the present study suggesting continued treatment is required to maintain TRT associated benefits.
TRT has been available since 1939. Concerns have been raised with regard to the safety of TRT in men with suggestions that it may stimulate the growth of androgen-dependent tumors to advance or cause prostate cancer. Although controlled studies which report the safety of testosterone therapy in men with prostate cancer are currently lacking, the limited available evidence suggests that such treatment may not pose an undue risk of prostate cancer recurrence, progression or development [Citation27]. Prostate volume and PSA measures were increased with TRT in patients in the present study. Without the use of a placebo-controlled group, we cannot ascertain whether the increase in PSA and prostate volume was significantly above that of the normal aging population. Whilst, the incidence of prostate cancer is not unexpected in an elderly cohort of patients, previous studies suggest that testosterone therapy does not increase the risk of prostate cancer though this information should be continued to be captured through long-term, controlled trials [Citation28]. Clinical practice guidelines [Citation29] were followed throughout this study with vigilant monitoring of such prostate health parameters at baseline and subsequent treatment visits. No patient exceeded the safety levels and no complications such as acute urinary retention or prostate surgery were reported throughout the study treatment period. Interruption of TRT did halt prostate volume increases and reduce PSA levels, suggesting a direct treatment effect on these parameters. Prostate symptoms and residual voiding volume, however, were improved with treatment and significantly regressed during the interruption, indicating that patient risk-benefit should be considered in cases of TRT and withdrawal. In fact, during TRT interruption four patients had suffered acute urinary retention, two underwent TURP and one patient underwent TURP due to progressive severe obstructive symptoms, although the causality of these features could not be determined. Large, controlled, long-term outcome studies are required to provide further evidence on TRT safety. Importantly, TRT has not been associated with clinically significant increases in PSA or an increased risk of prostate cancer, and TRT is associated with a lower risk of worsening LUTS in some studies [Citation30].
The present study has a few limitations. This was an observational study with no placebo-controlled group and, therefore, did not allow direct effects of treatment versus non-treatment to be compared. However, this was not the primary focus of the study. Furthermore, ethical issues of not treating hypogonadal men who presented at our clinic would be raised. Whilst there is a justification for further prospective randomized controlled studies, the large patient cohort and long-term follow-up period of up to 11-years permits clinically meaningful data ().
Figure 1. Hypogonadal men (mean age 59.5) underwent testosterone replacement therapy (TRT) for a maximum of 11 years. Group A had TRT interrupted for approximately 1.5 years and resumed thereafter. Group B were treated continuously. Prior to interruption, TRT resulted in improvements in residual voiding volume, bladder wall thickness, C-reactive protein (CRP), aging male symptoms (AMS), International Index of Erectile Function (IIEF-EF) and International Prostate Symptoms Scores (IPSS) and obesity parameters while prostate-specific antigen (PSA) and prostate volume increased. TRT interruption reduced total-testosterone to hypogonadal concentrations in Group A and resulted in worsening of obesity parameters, AMS, IPSS, residual voiding volume and bladder wall thickness, IIEF-EF and PSA while CRP and prostate volume were unchanged until treatment resumed whereby these effects were reversed. In Group B, parameters continued to improve throughout or remained the same during the equivalent interruption and post-interruption periods. Group A, n = 147; Group B, n = 115.
These data demonstrate that interruption of TRT resulted in worsening of symptoms in hypogonadal men, and that hypogonadism may therefore require lifelong treatment to maintain the observed benefits.
Acknowledgements
Editorial support for this manuscript was provided by Astra-Health, www.astra-health.co.uk.
Declaration of interest
Aksam Yassin has received partial compensation for data entry and occasional honoraria from Bayer Pharma, Ferring Pharmaceuticals and GSK. Gheorghe Doros has received payment for statistical analyses from Bayer Pharma. Joanne Nettleship, Raidh Talib and Yousef Almehmadi have nothing to disclose.
References
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015;18:5–15
- Wang C, Nieschlag E, Swerdloff RS, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2009;12:5–12
- Brooke JC, Walter DJ, Kapoor D, et al. Testosterone deficiency and severity of erectile dysfunction are independently associated with reduced quality of life in men with type 2 diabetes. Andrology 2014;2:205–11
- Donnell RF. Benign prostate hyperplasia: a review of the year’s progress from bench to clinic. Curr Opin Urol 2011;21:22–6
- Schatzl G, Madersbacher S, Haitel A, et al. Associations of serum testosterone with microvessel density, androgen receptor density and androgen receptor gene polymorphism in prostate cancer. J Urol 2003;169:1312–5
- Yassin DJ, El Douaihy Y, Yassin AA, et al. Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year prospective, observational and longitudinal registry study. World J Urol 2014;32:1049–54
- Rohrmann S, Smit E, Giovannucci E, Platz EA. Associations of obesity with lower urinary tract symptoms and noncancer prostate surgery in the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2004;159:390–7
- Seim A, Hoyo C, Ostbye T, Vatten L. The prevalence and correlates of urinary tract symptoms in Norwegian men: the HUNT study. BJU Int 2005;96:88–92
- Laven BA, Orsini N, Andersson SO, et al. Birth weight, abdominal obesity and the risk of lower urinary tract symptoms in a population based study of Swedish men. J Urol 2008;179:1891–5
- De Nunzio C, Aronson W, Freedland SJ, et al. The correlation between metabolic syndrome and prostatic diseases. Eur Urol 2012;61:560–70
- Kelly DM, Jones TH. Testosterone and obesity. Obes Rev 2015;16:581–606
- Schroeder ET, Zheng L, Yarasheski KE, et al. Treatment with oxandrolone and the durability of effects in older men. J Appl Physiol (1985) 2004;96:1055–62
- Schroeder ET, Zheng L, Ong MD, et al. Effects of androgen therapy on adipose tissue and metabolism in older men. J Clin Endocrinol Metab 2004;89:4863–72
- Corrales JJ, Almeida M, Miralles JM, Orfao A. Persistence of androgenic effects on the production of proinflammatory cytokines by circulating antigen-presenting cells after withdrawal of testosterone treatment in aging type 2 diabetic men with partial androgen deficiency. Fertil Steril 2009;92:311–9
- Francomano D, Bruzziches R, Barbaro G, et al. Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: a pilot study. J Endocrinol Invest 2014;37:401–11
- Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab 2011;96:2430–9
- Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring) 2013;21:1975–81
- Yassin A, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clin Obes 2013;3:73–83
- Francomano D, Ilacqua A, Bruzziches R, et al. Effects of 5-year treatment with testosterone undecanoate on lower urinary tract symptoms in obese men with hypogonadism and metabolic syndrome. Urology 2014;83:167–73
- Francomano D, Lenzi A, Aversa A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol 2014;2014:527470
- Haider A, Saad F, Doros G, Gooren L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational study. Obes Res Clin Pract 2014;8:e339–49
- Saad F, Yassin A, Doros G, Haider A. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes (Lond) 2015. [Epub ahead of print]. doi:10.1038/ijo.2015.139
- Yassin A, Almehmadi Y, Saad F, et al. Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clin Endocrinol (Oxf) 2015. [Epub ahead of print]. doi:10.1111/cen.12936
- Yassin AA, El-Sakka AI, Saad F, Gooren LJ. Lower urinary-tract symptoms and testosterone in elderly men. World J Urol 2008;26:359–64
- Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med 2014;11:1567–76
- Giltay EJ, Haider A, Saad F, Gooren LJ. C-reactive protein levels and ageing male symptoms in hypogonadal men treated with testosterone supplementation. Andrologia 2008;40:398–400
- Morgentaler A. Testosterone therapy in men with prostate cancer: scientific and ethical considerations. J Urol 2013;189:S26–33
- Haider A, Zitzmann M, Doros G, et al. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median followup of 3 registries. J Urol 2015;193:80–6
- Dohle GR, Arver S, Bettocchi C, et al EAU Guidelines on Male Hypogonadism. Available from: http://uroweb.org/guideline/male-hypogonadism [last accessed 25 Jun 2015]
- Grober ED. Testosterone deficiency and replacement: myths and realities. Can Urol Assoc J 2014;8:S145–7
