Abstract
Objectives: To investigate the predictive values of free prostate-specific antigen (fPSA), total PSA (tPSA) and age on the prostate volume.
Methods: The data of 2148 patients with lower urinary tract symptoms were analyzed retrospectively. The patients who had transrectal ultrasonography guided 10 core biopsies owing to the findings obtained on digital rectal examination and presence of high PSA levels (PSA = 2.5–10 ng/dl), and proven to have BPH histopathologically were included in the study. Age, tPSA, fPSA and the prostate volumes (PV) of the patients were noted.
Results: One thousand patients that fulfilled the inclusion criteria were included in the study. The PV of the patients were significantly correlated with age, tPSA and fPSA (p < 0.001 and r = 0.307, p < 0.001 and r = 0.382, p < 0.001 and r = 0.296, respectively). On linear regression model, fPSA was found as a stronger predictive for PV (AUC = 0.75, p < 0.001) when compared to age (AUC = 0.64, p < 0.001), and tPSA (AUC = 0.69, p = 0.013).
Conclusions: Although tPSA is an important prognostic factor for predicting PV, the predictive value of fPSA is higher. PV can easily be predicted by using age, and serum tPSA and fPSA levels.
Introduction
Baseline prostate volume (PV) is an important prognostic factor in determining the progression of benign prostatic hyperplasia (BPH). It was shown that PV enlargement was associated with hormonal changes. It has been reported that estrogens have been related to prostate hyperplasia in animal studies [Citation1,Citation2] and oestrogen levels increase with obesity by conversion of androgens in adipose tissue. Prostate volume significantly associated with insulin-like growth factor binding protein 3 (IGF-BP3) levels independent of age and body mass index [Citation3]. More than these, patients with a higher dihydrotestosterone (DHT) levels were related to higher PV [Citation4]. Karazidiyanoglu et al. [Citation5] have demonstrated that testosterone therapy healed lower urinary tract symptoms (LUTS). In addition, after transurethral resection of the prostate (TUR-P) for patients with large BPH, administration of 5α reductase inhibitors for three years has been shown to improve PV, prostate-specific antigen (PSA) and LUTS [Citation6]. However, in patients with hypogonadism, testosterone therapy was claimed to have no deleterious effect on International Prostate Symptom Score (IPSS), and it did not change PV [Citation7].
Currently, the standard method recommended for measurement of PV is transrectal ultrasonography (TRUS) [Citation8,Citation9]. TRUS of the prostate has a diagnostic accuracy >80%. However, TRUS is an expensive method, and it is bothersome to the patients this is why it is not used routinely for the initial evaluation of the patients. Access to TRUS is not always possible in primary care settings to screen larger prostate with potential risks. As a result, we need an inexpensive, simple and rapid method to predict the PV in our daily practice. Digital rectal examination is an inexpensive and non-invasive method to estimate the size of the prostate and it is not associated with subsequent side effects, but the evaluation of prostate by DRE depends on experience. Small prostates may be assessed as bigger, and the big ones may be assessed as smaller [Citation10]. Underestimation of PV is common in individuals having a bigger prostate compared to smaller cases. PSA, either total or free, is a different tool to guess PV for years. PSA is present in small quantities in men with healthy prostate, but it is detected higher in amount with prostate cancer, BPH or some other diseases of the prostate. It is the most effective test for the early detection of prostate cancer. Recent studies showed that free PSA has advantages to total PSA in the prediction of prostate size. Some researchers recommend a combination of radiologic and biochemical results for a more precise estimation than evaluation of a single parameter. Triple assessment including TRUS, DRE and PSA was determined to estimate BPH with 97% accuracy that is also useful to differentiate BPH from prostate cancer [Citation11].
Serum PSA levels increase when normal prostate anatomy impaired by diseases like BPH, prostate cancer or prostatitis and it may also be increased by trauma to the prostate. PSA may be used to estimate prostate volume in men with LUTS. In the absence of direct measurement of prostate volume, serum PSA determination is an alternative tool to estimate prostate volume. Prostate volume and age is associated with higher PSA. So age-specific PSA reference range has been developed, hence more efficient and more specific prostate cancer screening is possible.
It has been known that PSA increases in correlation with PV [Citation12,Citation13]. Considering PV and PSA increase with age, we may suppose that there is a link between age, serum PSA and PV. In this study, our aim was to analyze the relationships between PV and serum total PSA (tPSA), free PSA (fPSA) and age in patients with histopathologically proven BPH.
Methods
The data of 2148 patients who admitted to Urology Department of Afyon Kocatepe University Medical Faculty and Bursa Sevket Yilmaz Education and Research Hospital’s Urology Outpatient Clinic with LUTS between April 2011 and May 2014 were analyzed retrospectively, after the approval of Local Ethics Committee was obtained. The patients who had TRUS-guided 10 core biopsies owing to the findings obtained on DRE and presence of high PSA levels (PSA = 2.5–10 ng/dl) and proven to have BPH histopathologically were included in the study. The patients with PCa, an age < 40 years, the ones used 5-alpha reductase or had surgery for BPH previously were excluded.
The ones who had a cystoscopy, rectal interventions (e.g. colonoscopy), prostate biopsy, acute prostatitis or urinary retention in the previous month were also excluded. TRUS-guided biopsies were performed by two experienced urologists (S.C. and I.K.).
The age, tPSA, fPSA and PV of the patients were noted. The PV of the patients were calculated by measuring three dimensions of the prostate by TRUS, and using ellipsoid formula (PV = height × width × length × 0.52). Serum PSA levels were measured using chemiluminescent microparticle immunoassay (CMIA) method. For prostate enlargement, a volume of 40 ml was considered as the cutoff value. A model was established with linear regression analysis to predict PV using age, tPSA and fPSA. In addition, receiver operating characteristic curve (ROC) analysis was used to determine the predictive values of age, tPSA and fPSA on PV.
SPSS software, version 15.0 (SPSS, Inc., Chicago, IL) was used for statistical analyzes. Visual (histogram and probability graphs) and analytical (Kolmogorov–Smirnov test) measures were used to determine conformance of the variables to a normal distribution. Descriptive analysis results were presented as means and standard deviations for normally distributed variables. The relationships between age, tPSA, fPSA and PV were determined by the Spearman/Pearson correlation tests. The independent effects of age, tPSA and fPSA on the PV were evaluated using a multivariate linear regression model. Receiver operating characteristics (ROC) curves were constructed to compare and evaluate the ability of tPSA, fPSA and age in the estimation of PV. Statistical significance was set at p <0.05.
Results
A total of 1148 patients who did not fulfil the inclusion criteria were excluded. The remaining 1000 patients had a mean age of 63.12 ± 8.12 (41–86) years, a mean PV of 55.82 ± 29.83 ml, a mean tPSA level of 4.87 ± 2.93 ng/dl and a mean fPSA level of 1.08 ± 0.78 ng/dl. There were 370 patients (37%) with PSA levels between 0 and 4 ng/dl, and 630 (63%) patients with PSA levels between 4 and 10 ng/dl. PV and age, tPSA and fPSA of the patients showed significant correlations (p < 0.001 and r = 0.307, p < 0.001 and r = 0.382, p < 0.001 and r = 0.296, respectively) ().
Table 1. Correlations between age, TPSA, fPSA and prostate volume.
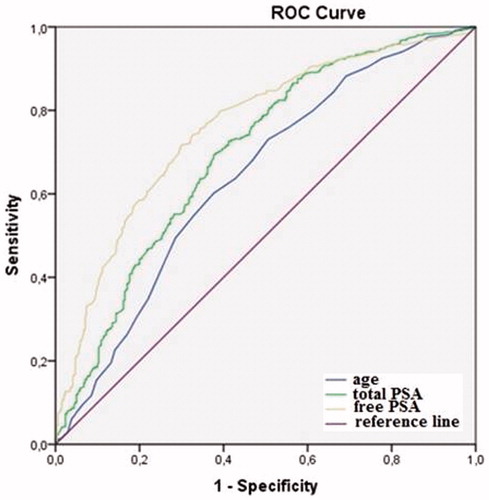
Free-PSA was found as a stronger predictor for PV (AUC = 0.75, p = 0.0001) in linear regression model when compared to age (AUC = 0.64, p < 0.001) and tPSA (AUC = 0.69, p = 0.013) (). The cutoff level for fPSA was determined as 0.775 ng/ml (specificity 65.6% and sensitivity 75.6%) ().
Table 2. Area under curve estimates from receiver operating characteristic curves predicting prostate volüme >40 ml.
Using linear regression model, a simple formula was developed: PV = 8.009 + (age × 0.473) + (tPSA × 0.984) + (fPSA × 12.18). According to this formula, the PV of a 40-year-old patient with a tPSA of 3 ng/ml and fPSA of 0.6 ng/ml is calculated as 37.18 ml.
Discussion
Prostate volume is important to predict the treatment outcomes and acute urinary retention in patients with BPH [Citation14–17]. BPH is a progressive disease, and the size of the prostate is important for prognosis [Citation18]. In MTOPS study, patients with a PV >30 ml were included in the study and followed up for a long time, and it was reported that PV was important for disease progression in the placebo group [Citation19]. Therefore, identifying PV is important for the follow-up of LUTS caused by BPH.
The standard method used for calculating PV is TRUS [Citation8,Citation9]. However, it is not reasonable to use this method in daily practice since it is an expensive method, and it is bothersome for the patients. In addition, it is not recommended in the routine evaluation armamentarium of BPH patients. DRE, which is used for predicting PV, may assess small prostates as bigger, and the big ones as smaller this is why we need a reliable, inexpensive and non-bothersome method to predict the PV in our daily practice.
PSA has been used in urology for diagnosis and treatment of prostate diseases since 1986 [Citation20]. A log-linear relation was shown between PSA and PV [Citation21]. Bohnen et al. [Citation22] reported that PSA was superior to DRE for predicting PV. Another study performed on American men found the correlation coefficient between PSA and PV as 0.39 [Citation13]. Similarly, in our study, we found a strong correlation between PSA and PV (r = 0.41).
Although some studies investigated the relation between tPSA and PV in patients with BPH, the relation between fPSA and PV has been less emphasized. Choi et al. showed that both tPSA and f PSA were statistically significantly correlated with PV. The ROC curves PV >30, >40 and >50 ml showed that AUC of fPSA were 0.781, 0.718 and 0.700, respectively. The authors stated that fPSA was more powerful for predicting PV [Citation23]. A recent study by Kayıkcı et al. reported that fPSA was as powerful as tPSA for predicting PV, and fPSA was even more powerful than tPSA in patients with a PV < 40 ml. The authors emphasized that a volume of 40 ml was important for starting 5α reductase treatment [Citation24]. Mao et al. showed that fPSA was a strong predictor for PV. The authors hypothesized that fPSA was more strongly correlated with PV when compared to tPSA, and the increase in fPSA was correlated better with BPH [Citation25].
Free/total PSA ratio has been used in addition to PSA levels and the physical examination findings in patients with a PSA level of 2.5–10 ng/dl, to differentiate between PCa and BPH, and to decide on a biopsy. A multicenter study determined PCa in 56% of the patients when f/t PSA < 0.10, and in 8% of the patients when it is > 0.10 [Citation26]. Those studies indicate that the probability of BPH increases as fPSA levels increase. Therefore, fPSA is more closely related to BPH.
Similarly, in our study, we found a higher correlation coefficient between fPSA and PV when compared to the one between tPSA and PV (r = 0.527, p <0.001 versus r = 0.41 p < 0.001). In addition, we showed that fPSA was superior to tPSA for predicting PV (AUC = 0.75 versus AUC = 0.69). When 0.775 was considered as the cutoff value for fPSA, the probability of PV to be > 40 ml was found as 75.6%.
In our study, we developed a simple formula using a linear regression model for predicting PV in daily practice by using age, tPSA and fPSA. We suppose that this simple formula can be used easily in the daily practice by incorporating it into the computer programs, and PV can be determined in an inexpensive way instead of using TRUS, which is bothersome for patients and not suitable for daily practice.
Our study is one of the few studies performed on a large patient cohort, and it has shown that fPSA is a strong predictor for PV. In addition, only patients with histopathologically proven BPH and had a gray-zone PSA level (<10 ng/dl) were included in the study in order not to raise any bias. However, the retrospective nature of our study and probability of occult cancers that could not be detected by biopsy are the limitations of our study.
Conclusions
It is important to know PV in BPH patients to administer them a proper treatment and follow them up appropriately. PV can simply be predicted by determining fPSA level in serum. We found that the correlation between fPSA and PV is stronger although there is a statistically significant correlation between tPSA and PV. In addition, PV can be correctly predicted by using a simple formula using fPSA, age and tPSA, in an inexpensive way.
Declaration of interest
The authors declare no conflict of interest.
References
- Farnsworth WE. Estrogen in the etiopathogenesis of BPH. Prostate 1999;41:263–74
- Coffey DS. Similarities of prostate and breast cancer: evolution, diet, and estrogens. Urology 2001;57:31–8
- Wallner LP, Clemens JQ, Sarma AV. Prevalence of and risk factors for prostatitis in African American men: the Flint Men’s Health Study. Prostate 2009;69:24–32
- Liao CH, Li HY, Chung SD, et al. Significant association between serum dihydrotestosterone level and prostate volume among Taiwanese men aged 40-79 years. Aging Male 2012;15:28–33
- Karazindiyanoğlu S, Cayan S. The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male 2008;11:146–9
- Qian X, Yu G, Qian Y, et al. Efficacy of 5α-reductase inhibitors for patients with large benign prostatic hyperplasia (>80 mL) after transurethral resection of the prostate. Aging Male 2015;18:1–6
- Meuleman EJ, Legros JJ, Bouloux PM. Study 43203 Investigators, et al. Effects of long-term oral testosterone undecanoate therapy on urinary symptoms: data from a 1-year, placebo-controlled, dose-ranging trial in aging men with symptomatic hypogonadism. Aging Male 2015;18:157–63
- Terris MK, Stamey TA. Determination of prostate volume by transrectal ultrasound. J Urol 1991;145:984–7
- Trop-Pedersen S, Juul N, Jakobsen H. Transrectal prostatic ultrasonograpyh: equipment, normal findings, benign hyperplasia and cancer. Scand J Urol Nephrol Suppl 1988;107:19–25
- Bosch JL, Bohnen AM, Groeneveld FP, et al. Validity of three calipler-based transrectal ultrasound methods and digital rectal examination in the estimation of prostate volume and its changes with age: the Krimpen study. Prostate 2005;62:353–63
- Trivedi MR, Choudhary BA. Digital rectal examination, transrectal ultrasound and prostate specific antigen as a triple assessment diagnostic tool for benign enlargement of prostate. Natl J Med Res 2015;5:244–8
- Roehrborn CG, Boyle P, Gould AL, et al. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Urology 1999;53:581–9
- Hochberg DA, Armenakas NA, Fracchia JA. Relationship of prostate-specific antigen and prostate volume in patients with biopsy proven benign prostatic hyperplasia. Prostate 2000;45:315–19
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol 1997;158:481–7
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol 1999;162:1301–6
- Bosch JL, Bohnen AM, Groeneveld FP. Validity of digital rectal examination and serum prostate specific antigen in the estimation of prostate volume in community-based men aged 50 to 78 years: the Krimpen study. Eur Urol 2004;46:753–9
- Marberger MJ, Andersen JT, Nickel JC, et al. Prostate volume and serum prostatic-specific antigen as predictors of acute urinary retention: combined experience from three large multinational placebo-controlled trials. Eur Urol 2000;38:563–8
- Kaplan SA, Roehrborn CG, McConnell JD, et al. Long-term treatment with finasteride results in a clinically significant reduction in total prostate volume compared to placebo over the full range of baseline prostate sizes in man enrolled in the MTOPS trial. J Urol 2008;180:1030–2. Discussion 1032–3
- Kaplan SA, Lee JY, Meehan AG. MTOPS Research Group, et al. Long-term treatment with finasteride improves clinical progression of benign prostatic hyperplasia in men with an enlarged versus a smaller prostate: data from the MTOPS trial. J Urol 2011;185:1369–73
- Smith DS, Catalona WJ, Herschman JD. Longitudinal screening for prostate cancer with prostate-specific antigen. JAMA 1996;276:1309–15
- Boyle P, Gould AL, Roehrborn CG. Prostate volume predicts outcome of treatment of benign prostatic hyperplasia with finasteride: meta-analysis of randomized clinical trials. Urology 1996;48:398–405
- Bohnen AM, Groeneveld FP, Bosch JL. Serum prostate-specific antigen as a predictor of prostate volume in the community: the Krimpen study. Eur Urol 2007;51:1645–52
- Choi H, Park JY, Shim JS, et al. Free prostate-specific antigen provides more precise data on benign prostate volume than total prostate-specific antigen in Korean population. Int Neurourol J 2013;17:73–7
- Kayikci A, Cam K, Kacagan C, et al. Free prostate-specific antigen is a better tool than total prostate-specific antigen at predicting prostate volume in patients with lower urinary tract symptoms. Urology 2012;80:1088–92
- Mao Q, Zheng X, Jia X, et al. Relationships between total/free prostate-specific antigen and prostate volume in Chinese men with biopsy-proven benign prostatic hyperplasia. Int Urol Nephrol 2009;41:761–6
- Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA 1998;279:1542–7