Abstract
This study analyzed the effects of dutasteride on lower urinary tract symptoms based on the association between changes in the total testosterone (TT)/dihydrotestosterone (DHT) levels and total prostate volume (TPV) reduction. Sixty participants diagnosed with benign prostatic hyperplasia were given 0.5 mg of dutasteride daily for 52 weeks. Measures of TT and DHT levels, TPV and uroflowmetry were obtained before and after dutasteride treatment. Forty-three patients demonstrated a TPV reduction of ≥5% (Group 1), whereas the remaining 17 patients demonstrated a TPV reduction of <5% (Group 2). DHT suppression and DHT/TT ratio at baseline were significantly higher in Group 1 than Group 2. International Prostate Symptom Scores (IPSS) and uroflowmetry were significantly improved in both groups. In Group 2, nine patients demonstrated some improvement in IPSS (Group 2A), whereas eight did not (Group 2B). The rate of TT increase and improvement in voiding symptoms were significantly higher in Group 2A than Group 2B. Dutasteride-induced TPV reduction is dependent on individual 5-α reductase inhibitor activity. Some patients demonstrating smaller dutasteride-induced TPV reduction may experience an improvement in voiding symptoms owing to an increased level of testosterone.
Introduction
Dutasteride, a 5-α reductase inhibitor (5-ARI), is commonly used worldwide to treat benign prostatic hyperplasia (BPH) by reducing the prostatic volume. A randomized, double-blind, placebo-controlled, parallel-group study in Japan demonstrated that 0.5 mg dutasteride administered once daily for 52 weeks could contribute to a 25% reduction in the prostate volume and significant improvement in the International Prostatic Symptoms Score (IPSS) and maximum flow rate (MFR) among Japanese patients with BPH [Citation1]. Furthermore, dutasteride was observed to reduce the need for future BPH-related surgery and the risk of future urinary retention in addition to reducing the prostate size [Citation2]. However, physicians have occasionally observed cases that have experienced effective benefits pertaining to lower urinary tract symptoms (LUTS) without reducing the prostate volume. Some previous studies demonstrated that patients with some predictors such as smaller BPH, low PSA levels or severe intravesical prostatic protrusion had less benefit from 5-ARI therapy [Citation3,Citation4]. Therefore, not all patients with BPH respond well to dutasteride treatment. These differences of individual responses to dutasteride have not been fully understood.
This study was a prospective design, and our objective was to analyze the effect of dutasteride on LUTS based on the association between changes in the total testosterone (TT)/dihydrotestosterone (DHT) levels and total prostate volume (TPV) reduction. In addition, we examined the effectiveness of dutasteride in cases demonstrating a lower reduction of TPV.
Patients and methods
Study subjects
Sixty patients (mean age, 72.5 years) with a diagnosis of BPH were prospectively enrolled in the present study. Inclusion criteria were as follows: an age of ≥50, a clinical diagnosis of BPH, an IPSS of ≥8 points and a TPV of ≥20 ml measured by transrectal ultrasonography (TRUS) [Citation5]. Exclusion criteria for the study were as follows: previous use of antiandrogenic agents, finasteride, phosphodiesterase-5 inhibitors or testosterone agents within 6 months prior to enrollment in the study; a diagnosis of neurogenic bladder and a history of prostate cancer, psychiatric disorder or active systemic disease. For patients with a prostate-specific antigen (PSA) level of >4.0 ng/ml, it was the responsibility of the investigators to eliminate the presence of prostate cancer.
The study protocol was approved by Kanazawa University Hospital Institutional Review Board, and all subjects gave informed written consent before participation in the study. Finally, 60 patients were enrolled in this study, and all patients were given 0.5 mg of dutasteride once daily for 52 weeks. All the medications associated with voiding or storage functions of the lower urinary tract, including alpha-1 blockers and anticholinergic agents, remained unchanged over the study period.
Study protocol
Before and after dutasteride treatment, PSA, TT and DHT levels and TPV were measured in all patients by TRUS and each patient completed the IPSS and Overactive Bladder Symptom Score (OABSS) questionnaires. TT and DHT values were evaluated using blood serum collected between 09:00 and 11:00. Immediately after collecting the blood samples, separated serum samples were stored at −20 °C until assay, and DHT was measured by radioimmuno assay (Mitsubishi LSI Medience Corporation, Tokyo). In addition, MFR and voided volume (VV) were analyzed based on uroflowmetry, and post-voided residual (PVR) volume was measured by abdominal ultrasonographic examination at baseline and at 52 weeks post the initiation of dutasteride.
Statistical analysis
Following dutasteride administration, patients were divided into two groups according to the rate of TPV reduction: patients with a TPV reduction of ≥5% (Group 1) and patients with a TPV reduction of <5% (Group 2). In addition, the subjects in Group 2 were also divided into two sub-groups: subjects demonstrating improvement in IPSS (Group 2A) and those demonstrating no improvement in IPSS (Group 2B). All variables were compared between Groups 1 and 2 and further between subgroups 2A and 2B. The parameters of each group were compared using the Mann–Whitney test. For each group, the changes in parameters were compared using the Wilcoxon’s signed rank test. All statistical analyses were performed using SPSS™ version 17.0 Medical Model (SPSS Inc., Chicago, IL). In all analyses, a p values < 0.05 indicated statistical significance.
Results
The mean pretreatment TPV was 46.1 ± 21.6 ml, and using dutasteride, TPV was reduced by a mean of −0.210 ± 25.8%. Of 60 participants receiving dutasteride therapy, 43 (72%) experienced a TPV reduction of ≥5% (Group 1), whereas the remaining 17 (28%) patients (Group 2) demonstrated a TPV reduction of <5%. A comparison of parameters between Groups 1 and 2 at baseline demonstrated that serum DHT concentration and DHT/TT ratio at baseline were significantly higher in Group 1 than Group 2 (). There were no significant differences between the two groups in the other baseline characteristics.
Table 1. Comparison of patient characteristics between the two groups.
Following dutasteride administration for a period of 12 months, prostate adenoma (transitional zone) volume showed a significant decrease in Group 1 (p < 0.001), whereas the volume was not reduced in Group 2 (p = 0.237) (). However, IPSS and MFR were significantly improved in both groups after the dutasteride treatment for 12 months. In addition, Group 1 demonstrated a significant decrease in PVR (p = 0.0467) as well as a slight improvement in OABSS (p = 0.0695). DHT concentration showed a significant decrease from 0.805 ± 0.335 to 0.151 ± 0.112 ng/ml (p < 0.001) in Group 1 and that of 0.636 ± 0.159 to 0.186 ± 0.133 ng/ml in Group 2 (p < 0.001). However, DHT suppression rate in Group 1 was significantly higher (−65.3 ± 31.0%) than that in Group 2 (−46.9 ± 23.8%) (p = 0.0172). Furthermore, PSA level showed a significant decline in both groups, but its rate of decrease was significantly higher in Group 1 (−60.5 ± 14.5%) than Group 2 (−43.7 ± 27.4%) (p = 0.00186).
Table 2. Comparison of parameters at baseline and at the 12-month visit (52 weeks post-dutasteride initiation) within groups.
Of the 17 patients experiencing a TPV reduction of <5%, nine (53%) patients demonstrated some improvement in IPSS (Group 2A), whereas eight (47%) patients demonstrated no improvement in the subjective symptoms of LUTS (Group 2B). At the baseline, there were no significant differences between Groups 2A and 2B in any parameters other than OABSS. Group 2A was observed to have a significantly lower mean OABSS as compared with Group 2B (2.8 ± 2.7 versus 5.8 ± 3.7, p = 0.0391) (). About 12 months after the initiation of dutasteride therapy, the mean TT value showed a significant increase as compared with that observed at the baseline (3.82 ± 1.28 versus 5.06 ± 0.95 ng/ml, p = 0.00111) in Group 2A, and a slight, but not significant increase in the mean TT value was observed (4.61 ± 0.79 versus 4.99 ± 1.08 ng/ml, p = 0.194) in Group 2B (). An increase in the mean rate of TT value was significantly higher in Group 2A (p = 0.0257). Based on analysis of UFM, between baseline and at 12-month post-initiation of dutasteride treatment in the two groups, Group 2A demonstrated a significant improvement in MFR (p = 0.0137), whereas group 2B did not show significant changes. On the other hand, VV and PVR showed no significant changes in either the group 2A or group 2B. The voiding symptoms of BPH were predominantly improved in Group 2A (.
Figure 1. Changes in IPSS subdomains before and after dutasteride treatment were shown. The domains including question 1 (Incomplete Emptying), 3 (Intermittency), 5 (Weak Stream) and 6 (Straining) were significantly improved in group 2A (*significant difference).
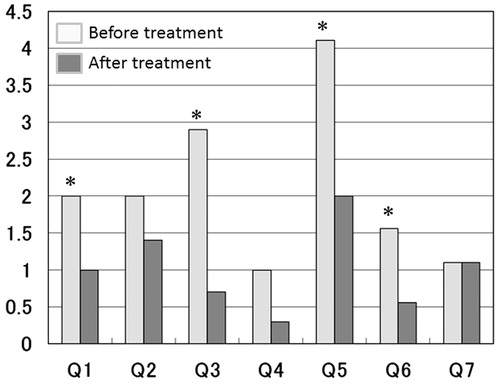
Table 3. Comparison of patient characteristics between the Group 2 sub-groups.
Table 4. Comparison of measured parameters between baseline and 12-month visit (52 weeks post-dutasteride therapy initiation) between the Group 2 sub-groups.
Discussion
Dutasteride has been available as an optional drug that acts by blocking the conversion of testosterone to DHT, thereby reducing the size of the prostate and in turn contributing to the improvement of LUTS for patients with BPH. Many previous large-scale studies have reported on the efficacy and safety of dutasteride for the treatment of BPH [Citation1,2,6–8]. Roehrborn et al. [Citation6] described that 569 patients had a mean reduction of −26.2 ± 22.0% in the prostate size following 48 months of dutasteride treatment. Another study demonstrated that dutasteride administration for >6 months contributed to a mean reduction of −21.0 ± 19.0% in the prostate size [Citation7]. In addition, a previous review mentioned that the mean prostate volume was reduced by −14.6 ± 13.5 cm3 following 24 months of dutasteride treatment. However, these values have a wide standard deviation for percent prostate reduction and this suggests that the effect of dutasteride on the clinical outcome of the prostate size reduction is highly variable [Citation8]. Currently, few studies are available to propose the reasons for the differences in individual patient response to dutasteride therapy. In the present study also, a TPV reduction of −0.210 ± 25.8% was observed following dutasteride administration for 12 months and 28% of the patients demonstrated a TPV reduction of <5%.
In the present study, Group 1 had a higher serum DHT concentration and DHT/TT ratio at baseline. In addition, the DHT suppression rate in Group 1 was significantly higher than that observed in Group 2. These findings suggest that the effect of dutasteride on TPV reduction may depend on individual 5-ARI activity, under the assumption that the DHT/TT ratio may indicate 5-ARI activity [Citation9,Citation10] and that the patients with a higher 5-ARI activity would experience benefits in addition to TPV reduction as a result of a higher level of DHT suppression.
Because DHT is a more potent androgen than testosterone, increased serum DHT concentrations can cause a greater prostate growth. Some studies have described that the serum DHT concentration and DHT/TT ratio were used clinically to monitor the 5-ARI inhibitor treatment of BPH, prevent prostate cancer and diagnose 5-ARI deficiency [Citation11,Citation12]. An experimental study used DHT/TT ratio to estimate 5-ARI activity in recurrent prostate cancer and benign prostate tissue and showed a difference of 5-ARI activity between prostate cancer and BPH [Citation12]. In addition, the expression level of SRD5A, a gene that codes 5-ARI, is elevated in BPH and prostate cancer, resulting in an enhanced production of DHT and overexpression of androgen receptors; it is also associated with the severity of BPH [Citation13,Citation14]. Therefore, any differences in individual 5-ARI activity may contribute to different responses to dutasteride.
However, both groups showed significant improvement in IPSS and MFR regardless of TPV reduction on dutasteride treatment. These findings suggest that the beneficial effects of dutasteride treatment on urinary symptoms are not always dependent on TPV reduction.
Some previous reports have investigated factors predicting the insufficient efficacy of dutasteride treatment for patients with LUTS and BPH. One of these studies found that patients with a TPV of <40 ml and low PSA levels experienced less benefit from 5-ARIs [Citation3]. Hirayama et al. [Citation4] described that a combination therapy of dutasteride and α1-blocker had insufficient efficacy in patients with LUTS/BPH associated with severe intravesical prostatic protrusion owing to a low proportion of stromal components. In the present study, there were no significant differences in pretreatment TPV or serum PSA levels between Groups 1 and 2. Our study population comprised patients with mild to moderate BPH, with a mean TPV of 46.1 ± 21.6 ml and included a relatively small number of severe BPH cases. In addition, intravesical prostatic protrusion of BPH was not investigated and additional studies are required to reach a more definite conclusion.
Furthermore, another unique report revealed a correlation between TPV reduction response to dutasteride treatment and androgen receptor (AR) activity [Citation7]. There was a greater reduction in the prostate volume in patients with a higher second to fourth digit ratio (≥0.95), and significant negative correlations were found between the digit ratio and TPV reduction rate. In general, a high activity of the androgen receptor increases the growth of the fourth digit, showing a lower digit ratio. The authors hypothesized that the response of the prostate to 5-ARI depends on AR activity, and a few residual DHT, not suppressed by dutasteride, bind to the androgen receptors that still have some effect on the prostate in the presence of a high androgen receptor activity.
We also conducted another sub-analysis on Group 2A and observed that 53% of these patients demonstrated a degree of improvement in IPSS and MFR, despite experiencing only a small TPV reduction. These results suggested that following dutasteride treatment, improvement in the subjective symptoms of LUTS was not always associated with TPV reduction. To our knowledge, this is the first study to analyze the urinary symptoms of the patients who lack TPV reduction.
Based on this sub-analysis, Group 2A was observed to have a significantly low baseline OABSS as compared with Group 2B. In addition, IPSS-questions 1, 3, 5 and 6 regarding voiding symptoms were predominately improved. For the cases with less reduction of TPV, dutasteride treatment may also be beneficial for patients who predominantly experience voiding symptoms as opposed to storage symptoms of BPH. Group 2A had a higher increase in the TT levels following dutasteride administration. Many recent studies describe that testosterone replacement could have some positive effects on LUTS in the patients with BPH [Citation15–22]. Amano et al. [Citation15] demonstrated that a TRT of testosterone ointment at a concentration of 6 mg/day administered for a period of 3 months improved LUTS and the AMS scores in 41 patients with LOH. A randomized controlled study noted that TRT improved IPSS score and uroflowmetry data among patients with LOH syndrome and mild BPH [Citation16]. Karazindiyanoğlu et al. [Citation17] mentioned that ART with transdermal testosterone gel for 1 year in the patients with LOH syndrome improved the IPSS, although mean prostate volume showed a significant increase. Furthermore, according to the results of pressure-flow analysis, this study demonstrated that ART significantly increased maximal bladder capacity and compliance and decreased detrusor pressure at maximal flow [Citation17]. These findings suggest that an increase in the TT levels following dutasteride administration has some favorable effects on LUTS for the patients belonging to group 2A.
Recently, many previous reports have demonstrated that TRT is an effective and safe treatment for aging men with LOH syndrome, and TRT can improve depressive symptoms, body weight, metabolic parameters and quality of life [Citation23–27]. In addition, an increase in TT following dutasteride administration can also have some add-on favorite effects on urinary function and general health, similar to that of TRT [Citation28,Citation29]. Further studies regarding these add-on effects of dutasteride are likely to be required to reach more definite conclusions.
In the present study, we could not identify a factor predictive of the beneficial effects of dutasteride in patients with BPH. Furthermore, DHT cannot be commonly used in clinical practice worldwide, and we cannot predict individual 5-ARI activity prior to treatment. Therefore, further studies are required to identify a useful predictor of the effectiveness of dutasteride.
Conclusion
Although TPV reduction induced by dutasteride treatment is likely to be dependent on individual 5-ARI activity, effective benefits may not always depend solely on TPV reduction. Patients with less TPV reduction using dutasteride may also experience benefits in their voiding symptoms as a result of an increase in testosterone levels subsequent to undergoing this therapy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Tsukamoto T, Endo Y, Narita M. Efficacy and safety of dutasteride in Japanese men with benign prostatic hyperplasia. Int J Urol 2009;16:745–50
- Roehrborn CG, Boyle P, Nickel JC, et al. ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002;60:434–41
- Lin VC, Liao CH, Wang CC, Kuo HC. 5α-Reductase inhibitor is less effective in men with small prostate volume and low serum prostatic specific antigen level. J Formos Med Assoc 2015;114:865–71
- Hirayama K, Masui K, Hamada A, et al. Evaluation of intravesical prostatic protrusion as a predictor of dutasteride-resistant lower urinary tract symptoms/benign prostatic enlargement with a high likelihood of surgical intervention. Urology 2015;86:565–9
- Homma Y, Kawabe K, Tsukamoto T, et al. Estimate criteria for diagnosis and severity in benign prostatic hyperplasia. Int J Urol 1996;3:261–6
- Roehrborn CG, Marks LS, Fenter T, et al. Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology 2004;63:709–15
- Kim TB, Oh JK, Kim KH, et al. Dutasteride, who is it more effective for? Second to fourth digit ratio and the relationship with prostate volume reduction by dutasteride treatment. BJU Int 2012;110:E857–63
- Marihart S, Harik M, Djavan B. Dutasteride: a review of current data on a novel dual inhibitor of 5alpha reductase. Rev Urol 2005;7:203–10
- Amaral C, Cunha SC, Fernandes JO, et al. Development of a new gas chromatography-mass spectrometry (GC-MS) methodology for the evaluation of 5α-reductase activity. Talanta 2013;107:154–61
- Gooren LJ, Saad F, Haide A, Yassin A. Decline of plasma 5alpha-dihydrotestosterone (DHT) levels upon testosterone administration to elderly men with subnormal plasma testosterone and high DHT levels. Andrologia 2008;40:298–302
- Wang C, Shiraishi S, Leung A, et al. Validation of a testosterone and dihydrotestosterone liquid chromatography tandem mass spectrometry assay: interference and comparison with established methods. Steroids 2008;73:1345–52
- Mohler JL, Gregory CW, Ford OH, III, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res 2004;10:440–8
- Das K, Lorena PD, Ng LK, et al. Differential expression of steroid 5alpha-reductase isozymes and association with disease severity and angiogenic genes predict their biological role in prostate cancer. Endocr Relat Cancer 2010;17:757–70
- Thomas LN, Douglas RC, Vessey JP, et al. 5alpha-reductase type 1 immunostaining is enhanced in some prostate cancers compared with benign prostatic hyperplasia epithelium. J Urol 2003;170:2019–25
- Amano T, Imao T, Takemae K, et al. Testosterone replacement therapy by testosterone ointment relieves lower urinary tract symptom in late onset hypogonadism patients. Aging Male 2010;13:242–6
- Shigehara K, Sugimoto K, Konaka H, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male 2011;14:53–8
- Kalinchenko S, Vishnevskiy EL, Koval AN, et al. Beneficial effects of testosterone administration on symptoms of the lower urinary tract in men with late-onset hypogonadism: a pilot study. Aging Male 2008;11:57–61
- Shigehara K, Namiki M. Late-onset hypogonadism syndrome and lower urinary tract symptoms. Korean J Urol 2011;52:657–63
- Takao T, Tsujimura A, Okuda H, et al. Lower urinary tract symptoms and erectile dysfunction associated with depression among Japanese patients with late-onset hypogonadism symptoms. Aging Male 2011;14:110–14
- Yassin DJ, El Douaihy Y, Yassin A, et al. Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year,prospective, observational and longitudinal registry study. World J Urol 2014;32:1049–54
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male 2016;19:1–6
- Saad F, Yassin AA, Haider A, Gooren L. Effects of testosterone on the lower urinary tract go beyond the prostate: new insights, new treatment options. Arab J Urol 2011;9:147–52
- Okada K, Yamaguchi K, Chiba K, et al. Comprehensive evaluation of androgen replacement therapy in aging Japanese men with late-onset hypogonadism. Aging Male 2014;17:72–5
- Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med 2014;11:1567–76
- Yassin A, Almehmadi Y, Saad F, et al. Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clin Endocrinol (Oxf) 2016;84:107–14
- Morgentaler A, Zitzmann M, Traish AM, Fox A. International expert consensus conference on testosterone deficiency and its treatment held in Prague, Czech Republic. Aging Male 2015;18:205–6
- Grosman H, Fabre B, Lopez M, et al. Complex relationship between sex hormones, insulin resistance and leptin in men with and without prostatic disease. Aging Male 2015. [Epub ahead of print]. doi: 10.3109/13685538.2015.1100600
- Shigehara K, Koh E, Sakamoto J, et al. Effects of dutasteride on lower urinary tract symptoms and general health in men with benign prostatic hypertroplasia and hypogonadism: a prospective study. Aging Male 2014;17:51–6
- Wada N, Hashizume K, Matsumoto S, Kakizaki H. Dutasteride improves bone mineral density in male patients with lower urinary tract symptoms and prostatic enlargement: a preliminary study. Aging Male 2015. [Epub ahead of print]. doi: 10.3109/13685538.2015.1072155
