Abstract
Introduction: Metabolic syndrome (MetS) is a constellation of interrelated risk factors of metabolic origin. Some studies suggest a possible link between low total testosterone (TT) levels and the presence of MetS.
Aim: To analyze the strength and independence of associations between TT and MetS components in non-diabetic men.
Methods: In this cross-sectional study, 143 non-diabetic men older than 40 were analyzed.
Main outcomes measure: Blood samples were collected to evaluate metabolic profile and TT levels. MetS was defined as the presence of three or more of the following characteristics: fasting blood glucose levels ≥ 100 mg/dL, triglyceride ≥ 150 mg/dL, HDL-c < 40 mg/dL, hypertension or blood pressure ≥ 130/85 mmHg, and waist girth > 102 cm.
Results: Mean age of the study population was 61.5 ± 8.61 years old. MetS was present in 47.9% of the individuals. Thirty-four men had low TT and MetS was observed in 23 (70%) against 50 (46%) in those with normal TT (≥ 300 ng/dL) (OR 4.94, p < 0.01), adjusted to confounder’s factors. In multiple linear regression analysis, only waist circumference (Beta: −0.395; p = 0.03) and HDL-c (Beta: 0.19; p = 0.04) remained significantly correlated with TT levels.
Conclusions: Low TT levels were associated with MetS diagnosis. Abdominal obesity was the MetS component independently correlated to low TT levels.
Introduction
Metabolic syndrome (MetS) is a constellation of interrelated risk factors of metabolic origin that appears to directly promote the development of atherosclerotic cardiovascular disease and type 2 diabetes mellitus (DM) [Citation1]. MetS affects approximately 25% of the adult population and its prevalence is increasing worldwide [Citation2,Citation3].
Studies in the literature have suggested that insulin resistance is the essential cause of the MetS [Citation4]. Although insulin-resistant individuals are not necessarily obese, they commonly have an abnormal fat distribution that is characterized by predominant upper body fat [Citation2]. Abdominal obesity is highly correlated with insulin resistance, thus other laborious measures of insulin resistance are currently unnecessary for the diagnosis of MetS [Citation5].
A large number of epidemiological studies have linked low total testosterone (TT) levels and its carrier protein, sex hormone-binding globulin (SHBG), to MetS and DM in men [Citation6–11]. However, the exact nature of the observed associations remains uncertain, given the high variability in the strength of the associations reported [Citation10]. Recent evidence suggests that associations may differ according to age and Body Mass Index (BMI), and stronger associations have been reported in young [Citation12] and non-obese [Citation13] men. In older non-diabetic men, low TT is associated with insulin resistance independently of central obesity [Citation11].
Aims
Considering these facts, the current study aims to evaluate the association between MetS diagnosis and low TT levels. Aside from that, correlations of the number of MetS components and TT levels and between the five individual clinical MetS components and TT levels were also explored. For this purpose, we analyzed the strength and independence of the associations between TT and MetS in groups of non-diabetic men older than 40.
Methods
Study population
The current study presents a cross-sectional design and included 143 men older than 40 who were referred to routine urological evaluation. The study protocol was approved by our local ethical committee, and all patients signed the written informed consent. Exclusion criteria included the presence of DM, heart failure, previous or present cancer, liver disease, previous pelvic surgery, testosterone replacing therapy and drugs that may influence the hypothalamic-pituitary-testis axis. Socio-demographic information was obtained and blood samples were drawn for biochemical and hormonal analysis. Height, weight, waist, hip circumference and blood pressure were measured using standard procedures.
Traditional cardiovascular risk factors, socioeconomic status, demographic data, lifestyle and previous morbidity were assessed using standardized questionnaires. The studied variables were age, smoking (more than 100 cigarettes in a lifetime period), present alcohol consumption and sedentary habits, defined as energy consumption less than 200 kcal/day and determined by using the International Physical Activity Questionnaire (IPAC short version) [Citation14].
Standardized blood pressure measurements were obtained with the patient sitting down and the average of three measurements were used in the analysis. Hypertension was defined by systolic blood pressure equal or above 135 mmHg, or diastolic blood pressure equal or above 85 mmHg, even the use of blood pressure-lowering drugs. Anthropometric assessment was performed with the patient wearing light clothing, no shoes, and the average of three measurements was used in the analysis.
Laboratory assays
Fasting blood samples were collected between 08 AM and 11 AM. Biochemical and hormone assays were performed at the Central Laboratory of Santa Casa de Misericórdia in Porto Alegre. TT was analyzed by chemiluminescent immunoassays (Elecsys 2010; Roche-Diagnostic, Germany), glucose, total cholesterol, HDL-c and triglyceride were analyzed by enzymatic assay. The normal range for the exams considered in this study was: glucose (70–99 mg/dL), total cholesterol (< 200 mg/dL), triglyceride (< 150 mg/dL), HDL-c (> 40 mg/dL) and TT (300–800 ng/dL). Triglyceride and HDL-c levels were adjusted for patients using statin, respectively, +15% and −5% [Citation15].
Statistical analysis and sample size
All the studied variables had normal distribution. Data are presented as mean ± standard deviation of the mean, unless otherwise stated. The sample was stratified into two groups, according to TT levels. Student t-test was used to compare continuous variables between low and normal TT groups. Chi-square test was used to compare categorical data. Multiple logistic regression analysis was used to control possible confounders.
Simple linear regression was used to measure correlation among continuous variables of MetS components and TT levels. Two multivariate linear regressions were carried out. The first multivariate linear regression was undertaken to explore TT levels and correlation with waist circumference, HDL-c, triglyceride, glucose and hypertension. The second multivariate linear regression was undertaken to evaluate the effect of MetS clinical components. Covariates included age, BMI, alcohol consumption, smoking and physical activity profile.
All statistical analyses were performed with IBM SPSS 20 (SPSS Inc., Chicago, IL). All statistical tests were two-sided and p < 0.05 were considered statistically significant. This study was designed to detect statically significant Pearson Correlation Coefficients of 0.3 or higher (moderated correlation), considering an alpha error of 5% and power of 90% and the sample size was calculated of at least 113 patients [Citation16].
Main outcome measures
Definition of MetS
MetS was defined according to the American Heart Association (AHA) and consisted in having three or more of the following characteristics: fasting blood glucose levels ≥ 100 mg/dL, serum triglyceride ≥ 150 mg/dL, serum HDL < 40 mg/dL, hypertension or blood pressure ≥ 130/85 mmHg and waist girth ≥ 102 cm [Citation2]. All parameters of MetS were analyzed as a continuous data, except hypertension that was evaluated as a categorical variable.
Definition of low TT levels
TT levels below which symptoms of androgen deficiency emerge and adverse health outcome ensue in older men remains unclear, and the use of arbitrary thresholds is not appropriate [Citation17]. Further analyses revealed nonlinear threshold relationships between sexual symptoms and TT at levels around or below the lower limit of eugonadal reference range for young men. Wu et al. suggest that the presence of at least three sexual symptoms and TT levels of less than 11 nmol per liter (320 ng/dL) can be defined as Late-onset hypogonadism [Citation18]. In this study, low testosterone levels were defined as TT lower than 300 ng/dL.
Results
A total of 143 patients were enrolled in this study. Mean age was 61.49 ± 8.61, 74.5% were Caucasian, 65.5% had already smoked more than 100 cigarettes in lifetime, 67.8% had diagnosis of hypertension or had high blood pressure at the time of evaluation, 47.9% had MetS, 41% were presently alcohol consumers, 62% were consider sedentary. Mean BMI was 28.36 kg/m2 ± 4.60, mean TT was 413.71 ng/dL ± 147.60, HDL-c 49.86 mg/dL ± 13.25, triglycerides 140.49 mg/dL ± 73.58 and glucose 96.75 mg/dL ± 14.40. depicts the clinical characteristics of the sample comparing those with low and normal testosterone.
Table 1. Clinical and demographic characteristics of the study population stratified for low (< 300 ng/dL) and normal (≥ 300 ng/dL) total testosterone.
Men with low TT levels were more likely to have prevalent MetS compared to men with normal TT levels (OR 6.03; IC 2,43–14,99; p < 0.01), adjusted for smoking, sedentary, age and present alcohol consumption. Association of MetS with low TT levels were attenuated but still statistically significant after including BMI in the multivariate regression (OR 4.94; IC 1,83–13,29; p < 0.01).
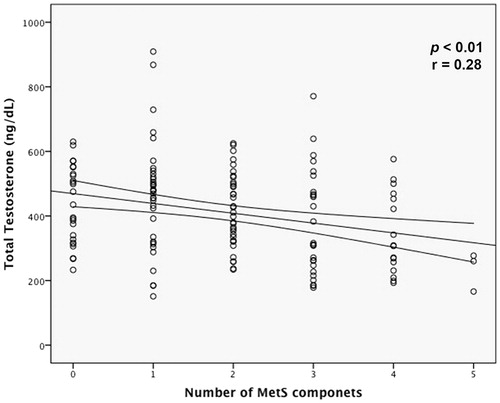
The number of clinical MetS components were inversely correlated with TT (R −0.28; p = 0.001) (). In a multiple linear regression analysis, the correlation between the number of MetS components and TT was independent of possible confounders factors (B −27.43; beta −0.26; p < 0.001). Moreover, each MetS components aggregation was associated with a reduction in TT levels of 27 ng/dL ().
Table 2. Multivariate correlation model between TT and the number of metabolic syndrome components.
When MetS components were analyzed separately, all but hypertension was correlated with TT levels. Triglyceride, glucose and waist circumference were inversely correlated with TT levels. HDL-c was directly correlated with TT levels. In the multiple linear regression analysis, only waist circumference and HDL-c persisted significantly correlated with TT (). Furthermore, each increased cm in waist circumference was associated with a reduction in TT levels of 5.25 ng/dL, independently of all other MetS components and BMI.
Table 3. Univariate and multivariate correlation between TT levels and individual MetS components.
Discussion
In this analysis of 143 non-diabetic men above 40, low TT levels were independently associated with MetS. Besides that, the number of MetS components was inversely correlated with TT levels. Considering the MetS components individually, waist circumference seems to be the strongest correlated factor to lower TT levels as demonstrated by multivariate analysis. The findings in this study corroborate the results found in previous studies [Citation19]. Previous studies have shown that associations of TT levels with MetS were strongest in non-overweight men and abdominal obesity and insulin resistance were the main drivers of the overall associations found [Citation11,Citation20]. Although insulin-resistant individuals do not need to be clinically obese, they nevertheless commonly have an abnormal fat distribution that is characterized by predominant upper body fat. A pattern of abdominal (or upper-body) obesity correlated more strongly to insulin resistance and MetS than did lower-body obesity [Citation21,Citation22].
The current study sought to specifically evaluate the linear correlation of MetS components individually with TT levels. Chubb et al. [Citation10] conducted a study that included 2502 older men (> 70) with and without MetS and they demonstrated that TT levels were associated with all MetS components, but for hypertension, in univariate analysis. In the current study, we have also found that all MetS components, but hypertension, were associated with lower TT levels in univariate analysis. These results confirm outcomes of previous studies [Citation10,Citation20,Citation23]. However, in multivariate analysis only waist circumference and HDL-c persisted significantly correlating with TT levels. Furthermore, we observed that TT levels were reduced in 5.25 ng/dL for each increase in waist circumference.
The cross-section design of the current study cannot draw definitive conclusions on the causal relationship of the observed associations. Interestingly, Brand et al. [Citation20], in a meta-analysis of cross-sectional and prospective studies, demonstrated a stronger association of sex hormones with prevalent than with incident MetS, suggesting that low TT levels are merely a result rather than a cause of MetS. Indeed, weight loss and maintenance have been associated with an increase in TT and SHBG levels in obese men with MetS [Citation24,Citation25]. Besides that, a prospective study with eugonadal patients showed that the presence of MetS had a 2.6 fold increased risk of developing TT levels lower than 11 mol/L on an 11-year follow-up period [Citation26].
On the other hand, prospective cohort studies suggested that low TT levels were a strong predictor for MetS incidence, particularly in non-overweight, middle-aged men [Citation12,Citation27]. Aside from that, some recent long-term uncontrolled prospective studies demonstrated that testosterone replacement therapy (TRT) improves MetS parameters [Citation28] and a meta-analysis of the few available testosterone supplementation studies confirmed that TRT was associated with a significant reduction of fasting glucose, HOMA-IR, triglycerides and waist circumference as well as an increase in HDL-cholesterol [Citation19]. Based on the above discussion, observational and experimental data suggest bidirectional relationship between TT levels and MetS in men.
The current study has certain limitations. The results are based on a single blood sample. Sex Hormone Binding Globulin and Bioavailable Testosterone Levels were not analyzed in this study. Although, the current study presents an adequate sample, from the perspective of statistics, this sample is small if compared with the huge collaborative studies [Citation10,Citation12,Citation29]. The patient profile analyzed in the study often use medications that might alter the values of some laboratory exams. Therefore, the full clinical information set allowed an analysis adjusted for statin use in the present study.
Conclusion
In conclusion, this cross-sectional study confirms a clear association between MetS and low testosterone levels in men. Besides that, a moderated correlation between TT levels and MetS components, especially the central feature of waist circumference in TT levels determination in non-diabetic patients. Apparently, a complex net links androgens, abdominal adiposity and MetS. Therefore, the male hormone profile assessment should be performed in patients with abdominal obesity, regardless of fill diagnostic criteria for MetS.
Declaration of interest
The authors report no declarations of interest. Ernani Rhoden participated as speaker or studies conducted by Besins healthcare, Astellas, Eli Lilly company, Bayer healthcare.
References
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–52
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–9
- Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin North Am 2004;33:283–303
- Carr DB, Utzschneider KM, Hull RL, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 2004;53:2087–94
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015;18:5–15
- Golan R, Scovell JM, Ramasamy R. Age-related testosterone decline is due to waning of both testicular and hypothalamic-pituitary function. Aging Male 2015;18:201–4
- Santeusanio F. Hypogonadism and the metabolic syndrome in men: an association to be considered. Nutr Metab Cardiovasc Dis 2008;18:253–5
- Rhoden EL, Ribeiro EP, Teloken C, et al. Diabetes mellitus is associated with subnormal serum levels of free testosterone in men. BJU Int 2005;96:867–70
- Chubb SA, Hyde Z, Almeida OP, et al. Lower sex hormone-binding globulin is more strongly associated with metabolic syndrome than lower total testosterone in older men: the Health in Men Study. Eur J Endocrinol 2008;158:785–92
- Yeap BB, Chubb SA, Hyde Z, et al. Lower serum testosterone is independently associated with insulin resistance in non-diabetic older men: the health in men study. Eur J Endocrinol 2009;161:591–8
- Haring R, Volzke H, Felix SB, et al. Prediction of metabolic syndrome by low serum testosterone levels in men: results from the study of health in Pomerania. Diabetes 2009;58:2027–31
- Kupelian V, Hayes FJ, Link CL, et al. Inverse association of testosterone and the metabolic syndrome in men is consistent across race and ethnic groups. J Clin Endocrinol Metab 2008;93:3403–10
- Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95
- LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA 1999;282:2340–6
- Hulley SB, Cummings SR, Browner WS, et al.. Designing clinical research. Philadelphia (PA): Wolters Kluwer/Lippincott Williams & Wilkins; 2013: 108
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2006;91:1995–2010
- Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123–35
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011;8:272–83
- Brand JS, Rovers MM, Yeap BB, et al. Testosterone, sex hormone-binding globulin and the metabolic syndrome in men: an individual participant data meta-analysis of observational studies. PLoS One 2014;9:e100409
- Jensen MD, Haymond MW, Rizza RA, et al. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest 1989;83:1168–73
- Amati F, Pennant M, Azuma K, et al. Lower thigh subcutaneous and higher visceral abdominal adipose tissue content both contribute to insulin resistance. Obesity (Silver Spring) 2012;20:1115–17
- Torkler S, Wallaschofski H, Baumeister SE, et al. Inverse association between total testosterone concentrations, incident hypertension and blood pressure. Aging Male 2011;14:176–82
- Niskanen L, Laaksonen DE, Punnonen K, et al. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004;6:208–15
- Kaukua J, Pekkarinen T, Sane T, et al. Sex hormones and sexual function in obese men losing weight. Obes Res 2003;11:689–94
- Laaksonen DE, Niskanen L, Punnonen K, et al. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. J Clin Endocrinol Metab 2005;90:712–19
- Kupelian V, Page ST, Araujo AB, et al. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab 2006;91:843–50
- Yassin AA, Saad F, Gooren LJ. Metabolic syndrome, testosterone deficiency and erectile dysfunction never come alone. Andrologia 2008;40:259–64
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007;92:4241–7