Abstract
Objective: To evaluate the effect of rosiglitazone on diabetic retinopathy in the usual care setting. Methods: Type 2 diabetic patients, aged over 40, who received rosiglitazone therapy for at least one year during the study period, were considered for the study group. All diabetic patients who had never received rosiglitazone or insulin were candidates for the control group. For each subject treated with rosiglitazone, up to five controls were randomly selected and matched on age, gender, and HbA1c%. We retrieved information about ophthalmologist visits, retinal argon laser therapy, vitrectomy and the date of the procedure. Time from the first rosiglitazone prescription to the first intervention was calculated. Results: A total of 6689 subjects, 1304 in the rosiglitazone group and 5385 in the control group were followed for a median of 3.6 years. The baseline level of HbA1C% was slightly higher in the rosiglitazone group (9.2 versus 8.8). There were more ophthalmologist visits in the rosiglitazone group compared to the control group. 115/1304 (8.8%) patients in the study group had an event compared to 379/5385 (7.0%) control group (P = 0.027). HR for the study group was 1.3 (95% CI: 1.08–1.64) for any event compared to control group (P = 0.008).
Conclusion: Rosiglitazone was associated with increased laser treatments and vitrectomy. Caution may be needed when treating diabetic patients with rosiglitazone.
Introduction
Long-term complications of diabetes mellitus have major impact on the quality of life of diabetic patients and carry a great economic burden. Diabetic retinopathy is the leading cause of blindness among adults aged 20–74 years in the US (Citation1). It is a leading cause of blindness in Israel, and its prevalence is increasing (Citation2).
The DCCT and the UKPDS trials have demonstrated the significance of glycemic control in preventing diabetes complications including diabetic retinopathy (Citation3,Citation4). Moreover, the effect of good glycemic control was noted even four years after the trial was ended (Citation5).
Rosiglitazone was introduced about a decade ago for treatment of hyperglycemia associated with diabetes mellitus. In experimental models, thiazolidinediones inhibit retinal neovascularization (Citation6). However, thiazolidinedione use was associated with fluid retention in 5–15% of patients and with macular edema in some patients, while drug discontinuation was related to resolution of the edema (Citation7).
Lately, it has been reported that rosiglitazone was associated with delayed onset of proliferative diabetic retinopathy (Citation8). We aimed to evaluate the effect of rosiglitazone, the only glitazone available in Israel, on diabetic retinopathy in the usual care setting. We chose interventions such as retinal photocoagulation or vitrectomy as endpoints, since they represent a significant diabetic retinopathy and are only performed after a retinal specialist has determined the need for such treatment.
Methods
We performed a community based, retrospective, cohort study using a nested approach to match between patients exposed to rosiglitazone and controls. We used automated clinical data from the Central District of Clalit Health Services (CHS) Israel, between 2002 and 2007. The institutional review board approved this study.
Study population
CHS is the largest health maintenance organization (HMO) in Israel serving more than 50% of the Israeli population. The Central District of CHS served a population of about 500 000 persons during the study period.
The source population consisted of all patients with type 2 diabetes mellitus as identified in the Diabetes Registry of the CHS aged 40 years or older, and filled prescriptions for at least one oral hypoglycemic agent in the year before their entry into the study.
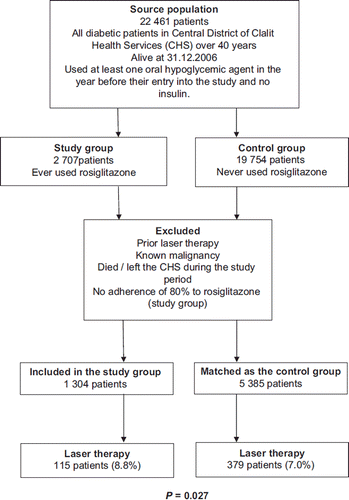
All patients who received continuous rosiglitazone therapy for at least one year during the study period were considered part of the rosiglitazone group. Only patients with adherence to rosiglitazone therapy of more than 80% for the prescribed drug were considered eligible. The cohort entry date for the study group was defined as the date of the first prescription for rosiglitazone.
Excluded from the study were subjects with one of the following conditions at baseline: insulin therapy, prior laser therapy and known malignancy as well as patients that died or left the HMO during the study period. All diabetic patients who never received rosiglitazone were candidates for the control group.
For each subject from the rosiglitazone group, up to five controls were randomly selected and matched on age, gender, and HbA1c%. For each control, the date of entry to study was the same as the matched rosiglitazone patient. For each subject we retrieved information about ophthalmologist visits and retinal argon laser therapy, vitrectomy and the date of the procedure. The time from the first rosiglitazone prescription to the first laser treatment was calculated.
Subject information
For each subject we retrieved socio-demographic, clinical and medication utilization data. Socio-demographic data included age, gender and socio-economic level. A patient was defined as having a low socio-economic status based on exemption from paying the monthly National Insurance dues. For each patient we also retrieved clinical information, including underlying medical conditions, and laboratory data. All laboratory studies were performed in the central regional laboratory of the Central District.
Setting
All community pharmacies in use by CHS are computerized and report to a central repository. All prescriptions of all brands of rosiglitazone, which were filled by the study population through CHS related pharmacies between 1 January 2002 (when rosiglitazone was put on the formulary of the CHS) and 31 December 2006, were documented. CHS dispenses medications with nominal and almost equal co-payment (between $3 and $8 for a monthly dosage).
During the study period, rosiglitazone was given to patients who did not achieve adequate control on metformin and/or sulfanilurea by the regulatory committee of CHS, reimbursement for rosiglitazone was restricted to patients with poor glycemic control by sulfonureas and/or metformin.
Endpoints
Routine, yearly, eye examinations of diabetic patients is performed by ophthalmologists in Israel. We retrieved information about all the ophthalmologist visits, retinal laser treatments and vitrectomy performed on the study cohort during the study period. Patients were censored by the date of first laser treatment or study termination.
Analysis
Treatment with rosiglitazone was the primary exposure of interest. We used Cox regression models to calculate the hazard ratio (HR) for retinal laser therapy after adjusting for patients’ socio-demographic factors, and laboratory data.
Values are reported as mean ± SD or percentages; 95% confidence intervals (CIs) are provided, when appropriate. All analyses were conducted using STATA 8.0 statistical software (Stata Corp. college Station, TX, USA) with two-tailed significance levels of 0.05.
Results
A total of 6689 subjects, 1304 in the rosiglitazone group and 5385 in the control group were followed for a median of 3.6 years. Patient eligibility is described in .
Baseline characteristics
The baseline characteristics of the patients in both groups are shown in . Participants in both groups were matched for age and gender. The baseline level of HbA1C% was slightly higher in the rosiglitazone group (9.2 versus 8.8, P < 0.001). There were no differences in blood pressure between the groups. The average LDL-cholesterol in the rosiglitazone group was lower compared to the control group. More patients in the control group had congestive heart failure (9.5% versus 6.1% P < 0.001) or a history of a cerebrovascular accident (11.2% versus 7.1% P < 0.001) compared to the rosiglitazone group. However, diabetes duration was similar in both groups.
Table I. Patients’ characteristics.
Endpoints
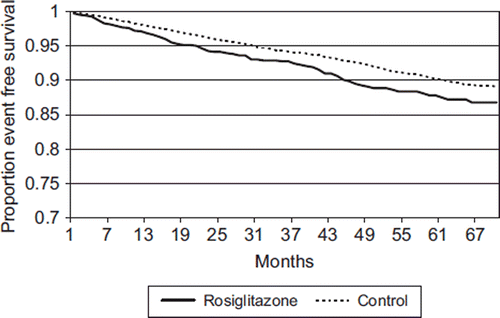
There were more ophthalmologist visits in the study group compared to the control group. In the study group, 115/1304 (8.8%) patients had an event (either laser therapy or vitrectomy) compared to 379/5385 (7.0%) in the control group (P = 0.027). The HR for the rosiglitazone group was 1.33 (95% CI: 1.08–1.64) for any event compared to the control group (P = 0.008). shows the time to laser treatment. Adjustment by Cox regression for age, gender, socioeconomic status, Hba1c, and ophthalmologist visits, did not alter the HR.
Discussion
Rosiglitazone use was associated with an increase in the need for retinal laser therapy among diabetic patients (HR 1.3).
Other studies
Little is known about rosiglitazone and its relation to diabetic retinopathy. Rosiglitazone may cause macular edema, which is reversible on drug discontinuation (Citation7,Citation10). However, Shen et al., who checked visual acuity changes among diabetic patients, found that fewer subjects in the rosiglitazone group experienced three or more lines of visual acuity loss (Citation8). In experimental models, rosiglitazone inhibits experimental retinal neovascularization (Citation9).
Strength and limitations
This study has several strengths. First, this is a large cohort of diabetic patients using rosiglitazone in the usual care setting for a relatively long period of follow-up. We could ascertain that the selected patients had good compliance with rosiglitazone, while the controls did not use rosiglitazone at all. Second, the fundus examination has a high penetration rate among this group; over 85% of them were seen by an ophthalmologist during the study period.
The blood pressure and LDL cholesterol levels of the rosiglitazone group were lower than that of the control group. The rosiglitazone group was less likely to have a history of congestive heart failure or cerebrovascular accident and fewer were from a low socioeconomic status. These differences probably represent a selection bias in the use of rosiglitazone. Since retinal involvement represents an advanced disease and poorer diabetes control, it seems reasonable to assume that patients in the rosiglitazone group, having similar diabetes duration and glycemic control, better blood pressure and LDL cholesterol levels, and fewer related diseases compared to control group, would need less invasive treatments for diabetic retinopathy.
The study's main limitation is related to the database we used. The data is based on hospital claims as the source information about photocoagulation. Therefore, we could not discriminate between the reasons leading to photocoagulation. Since rosiglitazone may cause macular edema, which is primarily treated with focal photocoagulation, this could explain the increase in retinal laser treatment we noted in the rosiglitazone group. Alternatively, rosiglitazone use has also been found to be related to a reduction in renal function (Citation11). These findings may represent a mechanism related to small blood vessel damage, which might cause diabetic retinopathy. Rosiglitazone use has been related to an increased risk of myocardial infarction and heart failure (Citation12). It is possible that another unknown mechanism might cause disease progression despite better glycemic control.
Diabetic retinopathy has no symptoms until its advanced stages. Laser photocoagulation and vitrectomy are not recommended until late stages of diabetic retinopathy. We chose to use laser photocoagulation as a marker for clinically significant disease progression because it represents clinically significant disease and because it can only be recommended by a retinal specialist. Though there might be differences among different ophthalmologists about when is the time for laser treatment, it always represents advanced disease or progression.
Practice implications
Our finding about the more rapid deterioration of diabetic retinopathy in the rosiglitazone group adds a new concern regarding rosiglitazone use. It reinforces the European Medicines Agency decision to suspend the use of rosiglitazone in the European Union (Citation13).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- CDC National Diabetes Fact Sheet, 2007. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf (accessed 12 May 2011).
- Avisar R, Friling R, Snir M, Avisar I, Weinberger D. Estimation of prevalence and incidence rates and causes of blindness in Israel, 1998–2003. Isr Med Assoc J. 2006;8:880–1.
- DCCT Research Group: The effects of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
- UKPDS Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–53.
- The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–9.
- Murata T, Hata Y, Ishibashi T, Kim S, Hsueh WA, Law RE, . Response of experimental retinal neovascularization to thiazolidinediones. Arch Ophthalmol. 2001;119:709–17.
- Ryan EH Jr, Han DP, Ramsay RC, Cantrill HL, Bennett SR, Dev S, . Diabetic macular edema associated with glitazone use. Retina 2006;26:562–70.
- Shen LQ, Child A, Weber GM, Folkman J, Aiello LP. Rosiglitazone and delayed onset of proliferative diabetic retinopathy. Arch Ophthalmol. 2008;126:793–9.
- Bosco AA, Lerario AC, Santos RF, Wajchenberg BL. Effect of thalidomide and rosiglitazone on the prevention of diabetic retinopathy in streptozotocin-induced diabetic rats. Diabetologia 2003;46:1669–75.
- Tatti P, Arrigoni F, Longobardi A, Costanza F, Di Blasi P, Merante D. Retrospective analysis of rosiglitazone and macular oedema in patients with type 2 diabetes mellitus. Clin Drug Investig. 2008;28:327–32.
- Feldman L, Shan MI, Efrati S, Beberashvili I, Baevsky T, Weissgarten J, . Association between rosiglitasone use and the decline in renal function in the patients with diabetes mellitus type 2. J Nephrol. 2010;23:350–6.
- Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–95.
- http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2010/09/WC500097003.pdf (accessed 12 May 2011).