Abstract
Venous thrombo-embolism (VTE, pulmonary embolism and deep vein thrombosis) is common in the elderly and short-term mortality risk increases with age. Hence, notably in older patients, accurately diagnosing VTE can be lifesaving. However, most clinically suspected individuals turn out to have no VTE after imaging examination. Therefore, many physicians would feel reluctant to refer older patients as this can be very burdensome for these patients. Consequently, it is possible that elderly patients are often not referred for diagnostic work-up (risk of under diagnosis), or that treatment for VTE is initiated without confirmation by further testing (risk of overtreatment). Moreover, anticoagulation treatment of VTE is associated with a higher bleeding risk in the elderly. This bleeding risk might even outweigh the potential benefits in some of these patients. Therefore, availability of an accurate diagnostic strategy to safely exclude, and timely diagnose VTE without the need of burdening referrals in many patients might better serve the needs of older patients. Such strategies have been derived and validated in both primary and secondary care patients suspected of VTE. However, the generalizability of these strategies to older patients may be hampered, and their accuracy has never been tested in elderly populations; this in spite of the high prevalence of VTE and the potential for misdiagnosis and thus mistreatment in these patients. Therefore, we advocate validation and adaptation of current diagnostic strategies for VTE for application in elderly patients.
KEY MESSAGE:
Diagnostic decision strategies for deep vein thrombosis (DVT) that are derived in adult patient populations may not be generalizable to older patients
Starting anticoagulation treatment without imaging in frail older patients with suspected DVT may result in more risk (major bleeding) than benefit (preventing pulmonary embolism) in some of these patients
INTRODUCTION
An everyday patient
Clinical scenario. After a busy morning, you are making a home visit to one of the oldest patients of your practice: a 98-year-old female who has problems walking. Her medical history documents hypertension, a decreased kidney function and a minor stroke several years ago. Since then, she uses acetylsalicylic acid. She called you because she suddenly had a red, swollen and painful leg. Fever is absent. You immediately consider the diagnosis of deep venous thrombosis (DVT). As the mortality of venous thrombo-embolism (VTE, pulmonary embolism or DVT) rises considerably with age, you realize that accurately and timely diagnosis of VTE is important (Citation1).
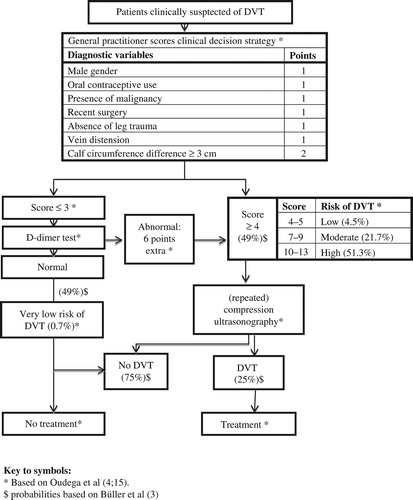
Diagnostic strategy. You decide to apply the primary care diagnostic decision strategy for DVT (), which yields a score of three points: there was no leg trauma, and measuring the calf circumference of both legs revealed a difference of 4 cm. In line with your national guideline, you perform a D-dimer test, as low D-dimer concentrations in combination with a low clinical probability would rule out DVT (Citation3,Citation4). However, the result is positive, leading to an extra six points on the diagnostic decision score, totalling it to nine points (moderate clinical probability of DVT; ). Consequently, you should refer the patient to the hospital for compression ultrasound examination (Citation3,Citation4).
Clinical dilemma. Since the accuracy of the diagnostic strategies for VTE has never been examined in very old patients, you are not sure whether this strategy can be translated to your 98-year-old patient (Citation5). Furthermore, you feel discouraged to refer this frail, old woman to a hospital for imaging investigation as this may stress her severely. Facing this dilemma, considering the possibility of DVT and the subsequent risk of fatal pulmonary embolism (PE) as high, you decide to not refer your patient for imaging, but instead to start anticoagulation treatment immediately. Back in your practice you discuss your dilemma concerning this patient with your colleagues. Was this the best possible decision? What about the risk of bleeding, one colleague argues?
In the current paper, we discuss the generalizability of diagnostic decision strategies for elderly patients, and the aims of those strategies versus the needs of elderly patients are discussed. Furthermore, we discuss whether it would be wise to start anticoagulation treatment in elderly patients with suspicion of DVT for whom imaging information is lacking because this examination is considered too burdensome.
GENERALIZABILITY OF DIAGNOSTIC DECISION STRATEGIES TO OLDER PATIENTS
Isolated signs and symptoms are not sufficient to diagnose or refute VTE (Citation2). Therefore, diagnostic decision strategies to exclude VTE—based on a weighed combination of signs, symptoms and D-dimer testing—have been derived, and their safety and cost-effectiveness has been tested in both primary and secondary care patients suspected of VTE (e.g. Wells strategy for PE and Oudega's strategy for DVT) (Citation3,Citation6–8). Those diagnostic decision strategies aim to help physicians in accurately and efficiently diagnosing or excluding VTE when applied in new suspected patients. Hence, the decision strategies need to be generalizable, that is, they should be able to produce accurate predictions among patients from a different but plausibly related population (Citation9). However, diagnostic models are sensitive to differences in patient populations, and often perform worse when applied in different patients (Citation9–10). For example, diagnostic models developed in a hospital setting may perform poorly in a primary care setting due to differences in patient characteristics, physicians experience and prior probabilities (Citation8–10). For your 98-year-old patient, the higher incidence of VTE and the often more obscure presentation of VTE in older patients may alter the diagnostic accuracy of the diagnostic models for DVT (Citation5,Citation12). Moreover, the D-dimer concentration increases with age, which may lead to a false positive D-dimer result in your patient (Citation11,Citation13). All these features may result in an over- or underestimation of the probability (an error in calibration), or in a reduced discriminative power between the presence or absence of DVT for your patient (Citation14). Hence, generalizability of diagnostic models for VTE—which are derived in adult patient populations—towards elderly patients, is questionable.
CURRENT DECISION STRATEGIES VERSUS THE NEEDS OF ELDERLY PATIENTS
One may also question whether the diagnostic decision strategies optimally serve the needs of elderly patients. The primary goal of the current diagnostic strategies for VTE is to provide the physician the assurance that VTE is not falsely excluded (Citation3). Therefore, in the current strategies, only very low proportions of false–negative results are deemed acceptable, and only in patients with a very low risk of VTE (commonly a threshold of < 1% to 4% is used) further imaging examination is withheld () (Citation6,Citation7).
Although primary care studies demonstrated that this safe ‘rule-out strategy’—encompassing a diagnostic decision rule and D-dimer testing—was able to exclude DVT in around 50% of patients, this strategy (still) led to a substantial proportion of patients being referred to a hospital. Three out of four patients who were referred for additional diagnostic work-up turned out to have no thrombosis (false–positive cases, ) (Citation3,Citation15). As the main aim of this strategy was to safely exclude DVT, this proportion of referred patients in whom no DVT was present, was deemed acceptable (Citation15).
However, for older patients the journey to a hospital and the undergoing of diagnostic research is often more burdensome and complicated (Citation16). Many physicians would feel reluctant to refer frail older patients to a hospital for exclusion of VTE and consequently might decide not to refer these patients for work-up despite an underlying ‘high’ risk of VTE (i.e. risk of under diagnosis) (Citation17). Alternatively, it is also possible that treatment for VTE is initiated in actually ‘low risk’ patients, thus without confirmation by further testing (i.e. risk of overtreatment).
THE BENEFIT-RISK RATIO OF ‘BLIND’ ANTICOAGULATION TREATMENT IN ELDERLY PATIENTS WITH SUSPICION OF DVT IN WHOM IMAGING EXAMINATION IS LACKING
If you consider referral for an imaging examination too burdensome for your 98-year-old patient, would it be wise to initiate anticoagulation treatment in this phase? In the current paragraph, we will roughly estimate and balance the potential risks and benefits of treatment against those of no treatment, based on available literature.
Assuming that the primary care diagnostic decision strategy is generalizable, your patient would have a probability of DVT being present of 21.7% (given her total score of nine, ) (Citation15). Hence, initiation of anticoagulation treatment would be useless in 4 out of 5 patients similar to our patient, whereas in 1 out of 5 cases, it may prevent prolongation of the thrombus and subsequently reduce the risk of potentially fatal PE. In 1959, Bauer described a case series of 29 untreated DVT patients; in 11 of these patients subsequently, PE occurred (38%) (Citation18). No studies on the natural history of idiopathic symptomatic VTE have been performed after 1960 (Citation19). Multiplying the probability of DVT (21.7%) with the probability of subsequent PE (38%) results in an estimated absolute risk of PE of 8.2% for our patient. Assuming that anticoagulation treatment would minimize this risk, one has to treat 12 patients with suspected DVT to prevent one case of PE (number needed to treat: 100 divided by 8.2).
The risk of prolongation of a thrombus can be balanced against the risk of major bleeding due to anticoagulation treatment for VTE, which increases with age and the presence of comorbidity. The HAS-BLED score—developed to estimate the bleeding risk in patients with anticoagulation treatment for atrial fibrillation—totals to five for your patient (hypertension, abnormal renal function, stroke, elderly and drugs (acetylsalicylic acid use), resulting in an estimated annual major bleeding risk of 12.5%, or a risk of 6.3% in the recommended six-months treatment period for VTE. Assuming a negligible bleeding risk in patients without anticoagulant treatment, this equals a number needed to harm of 16 (100 divided by an absolute increase in bleeding risk of 6.3; ) (Citation20,Citation21). As a comparator, patients without any risk factor on the HAS-BLED score–i.e. aged < 65 years and without relevant co-morbidity—would have an 11 times lower annual bleeding risk (1.13%).
Table 1. ‘Blind treatment’ in patients suspected of DVT (i.e. without confirmation by ultrasonography): clinical probability score for DVT against HAS-BLED. We recommend cautious interpretation of this consideration. First, though standard of care, the assumption that anticoagulant treatment would benefit the outcome of thrombosis is based on scarce and old data. Improved imaging techniques led to a substantial increase of the detection rates of smaller thrombi over the past decades which led to a doubling of the incidence. Hence, assumptions concerning the natural course of disease and the benefit of anticoagulation are derived from populations distinct from that being treated nowadays. For the latter, the clinical benefit of anticoagulation treatment might thus be over-estimated (Citation23). Second, we compared the complication risk of VTE with the major bleeding risk. However, the impact of major bleeding - especially intracranial bleeding - on the quality of life, mortality risk and healthcare costs- might be higher than the impact of pulmonary embolism (Citation24–26).
For patients like your 98-year-old woman with possible DVT, the number needed to treat (twelve) to prevent one case of PE is about the same as the number needed to harm (sixteen) to cause one case of severe bleeding, implying that initiating anticoagulation treatment possibly harms her as much as helps her.
However, her bleeding risk (6.3%) might be outweighed by her potential benefit of treatment if other possible complications are taken into account as well (such as less chance of prolongation of the thrombus towards proximal leg veins and lower probability of developing a post thrombotic syndrome), or if the diagnosis of DVT would be confirmed by imaging examination of the leg (Citation22).
IMPLICATIONS
Based on available although weak evidence, we should make the recommendation not to start anticoagulation treatment in elderly patients suspected of having DVT in whom the bleeding risk outweighs the clinical probability of PE, unless the diagnosis is truly confirmed by ultrasonography examination ().
Current available diagnostic strategies recommend referral for further imaging examination for 1 out of 2 patients with suspected VTE (), whereas diagnostic decision strategies that would spare a higher proportion of patients with suspected VTE the possible hazardous referral for imaging examination might better serve the needs of elderly patients. Therefore, we advocate validation and possibly subsequent adaptation of the current diagnostic strategies for elderly patients with suspected VTE by a so-called ‘updating study’ (Citation9). Diagnostic decision strategies should be tailored to the needs of older patients by recommending imaging examination in only a small proportion of them. This could be achieved by a two-track policy with both rule out and rule in strategies:
– The proportion of elderly patients in whom VTE can be safely excluded without imaging examination can be enlarged by application of age-adjusted D-dimer levels in elderly patients (Citation27,Citation28). Furthermore, a diagnostic decision strategy with the focus on improved efficiency (i.e. increasing the proportion of patients in whom DVT can be ruled out without the need for imaging examination, e.g. by applying a higher cut-off value) might be more appropriate in the elderly (inherently accepting that the proportion of falsely missed VTE cases also rises). Given the risks and burden involved with referral to a hospital in older patients, one could argue that applying the strict recommendation of missing a maximum of 1–4% of DVT cases, is too stringent for elderly care.
– If possible, a rule-in approach should be developed for older patients. With such an approach, patients with very high probabilities for VTE based on a diagnostic decision strategy are treated without further diagnostic imaging examination. Ideally, such a diagnostic decision strategy would incorporate thromboembolic complication risks as well as the major bleeding risks.
ACKNOWLEDGEMENTS
Contributors: HJS drafted the manuscript. HLK, RO, KGMM, JJMvD and GJG provided critical revision of the manuscript.
FUNDING
Financial support was provided by the Netherlands Organization for Scientific Research (ZonMw project number 17088-2502). This organization had no influence on any aspect of this study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- White RH. The epidemiology of venous thromboembolism. 2003;107(23 Suppl 1):14–8.
- Goodacre S, Sutton AJ, Sampson FC. Meta-analysis: The value of clinical assessment in the diagnosis of deep venous thrombosis. Ann Intern Med. 2005;143:129–39.
- Büller HR, Ten Cate-Hoek AJ, Hoes AW, Joore MA, Moons KG, Oudega R, et al. Safely ruling out deep venous thrombosis in primary care. Ann Intern Med. 2009;150:229–35.
- Oudega R, van Weert H, Stoffers HEJH, Sival PPE, Schure RI, Delemarre J, et al. NHG standaard diep veneuze trombose. Huisarts Wet. 2008;51:24–37.
- Siccama RN, Janssen KJ, Verheijden NA, Oudega R, Bax L, van Delden JJ, et al. Systematic review: Diagnostic accuracy of clinical decision rules for venous thromboembolism in elderly. Ageing Res Rev. 2011;10:304–13.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006;295:199–207.
- Geersing GJ, Erkens PM, Lucassen WA, Büller HR, Cate HT, Hoes AW, et al. Safe exclusion of pulmonary embolism using the Wells rule and qualitative D-dimer testing in primary care: Prospective cohort study. Br Med J. 2012;345:e6564.
- Oudega R, Moons KG, Hoes AW. Ruling out deep venous thrombosis in primary care. A simple diagnostic algorithm including D-dimer testing. Thromb Haemost. 2005;94:200–5.
- Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. Br Med J. 2009;338:b606.
- Reilly BM, Evans AT. Translating clinical research into clinical practice: Impact of using prediction rules to make decisions. Ann Intern Med. 2006;144:201–9.
- Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: A systematic review. Ann Intern Med. 2004;20;140:589–602.
- Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ, III. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch Intern Med. 1998;158:585–93.
- Harper PL, Theakston E, Ahmed J, Ockelford P. D-dimer concentration increases with age reducing the clinical value of the D-dimer assay in the elderly. Intern Med J. 2007;37:607–13.
- Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. Br Med J. 2009;338:b605.
- Oudega R, Moons KG, Hoes AW. Ruling out deep venous thrombosis in primary care. A simple diagnostic algorithm including D-dimer testing. Thromb Haemost. 2005;94:200–5.
- Gill TM, Gahbauer EA, Han L, Allore HG. The relationship between intervening hospitalizations and transitions between frailty states. J Gerontol A Biol Sci Med Sci. 2011;66:1238–43.
- Schouten HJ, van GS, Koek HL, Geersing GJ, Oudega R, Moons KG, et al. Non-diagnosis decisions and non-treatment decisions in elderly patients With cardiovascular diseases, do they differ? A systematic review. J Am Med Dir Assoc. 2012;13:682–7.
- Bauer G. The introduction of heparin therapy in cases of early thrombosis. Circulation 1959;19:108–9.
- Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. Lancet 1960;1: 1309–12.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro heart survey. Chest 2010;138:1093–100.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition). Chest 2008;133:454S–545S.
- Kachroo S, Boyd D, Bookhart BK, LaMori J, Schein JR, Rosenberg DJ, et al. Quality of life and economic costs associated with postthrombotic syndrome. Am J Health Syst Pharm. 2012;69:567–72.
- Prasad V, Rho J, Cifu A. The diagnosis and treatment of pulmonary embolism: A metaphor for medicine in the evidence-based medicine era. Arch Intern Med. 2012;172:955–8.
- Linkins L, O’Donnell M, Julian JA, Kearon C. Intracranial and fatal bleeding according to indication for long-term oral anticoagulant therapy. J Thromb Haemost. 2010;8:2201–7.
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: A meta-analysis. Ann Intern Med. 2003;139:893–900.
- Nieto JA, Solano R, Ruiz-Ribo MD, Ruiz-Gimenez N, Prandoni P, Kearon C, et al. Fatal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: Findings from the RIETE registry. J Thromb Haemost. 2010;8:1216–22.
- Douma RA, Le GG, Sohne M, Righini M, Kamphuisen PW, Perrier A, et al. Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: A retrospective analysis of three large cohorts. Br Med J. 2010;340:c1475.
- Schouten HJ, Koek HL, Oudega R, Geersing GJ, Janssen KJ, van Delden JJ, et al. Validation of two age dependent D-dimer cut-off values for exclusion of deep vein thrombosis in suspected elderly patients in primary care: Retrospective, cross sectional, diagnostic analysis. Br Med J 2012 Jun 6;344:ee2985.