Abstract
Background: European disease-specific antibiotic prescribing quality indicators (APQI) were proposed for seven acute indications (bronchitis, upper respiratory infection, cystitis, tonsillitis, sinusitis, otitis media and pneumonia): (a) the percentage of patients prescribed an antibiotic; (b) the percentage of patients receiving the guideline recommended antibiotic; (c) the percentage of patients receiving quinolones.
Objectives: To assess the feasibility of calculating values for these 21 APQI using primary care databases; and to assess the quality of antibiotic prescribing in office hours and out-of-hours general practice.
Methods: Data was extracted from a morbidity registration network (http://www.intego.be) and the out-of-hours service centre in Flanders. Within both databases diagnoses are labelled using the revised second edition of International Classification of Primary Care (ICPC-2-R) and antibiotic prescriptions using Anatomical Therapeutic Chemical (ATC) classification.
Results: Both databases allow calculation of APQI values and results are similar. Only for cystitis was the percentage of patients prescribed an antibiotic within the proposed acceptable range. For all indications, the percentage of recommended antibiotics was below the proposed acceptable range (80–100%). The percentage of quinolones was within the proposed acceptable range (0–5%) for otitis media, upper respiratory infection and tonsillitis.
Conclusion: Primary care databases can produce APQI values. These values revealed huge opportunities to improve the quality of antibiotic prescribing in office hours and out-of-hours Flemish general practice, especially the prescription of recommended antibiotics.
KEY MESSAGE:
Application of disease-specific antibiotic prescribing quality indicators (APQI) is feasible for databases linking diagnosis and prescription data.
Assessment of APQI revealed suboptimal quality of antibiotic prescribing in office hours and out-of-hours Flemish general practice.
Using recommended antibiotics offers a huge opportunity for quality improvement.
INTRODUCTION
Antibiotic use is increasingly recognised as the main driver for antimicrobial resistance (Citation1–3). The largest volumes of antibiotic prescriptions for systemic use are prescribed in primary care (Citation1), with respiratory (RTI) and urinary tract infections (UTI) being the most common indications (Citation4). If we want to improve antibiotic use, we have to be able to measure its quality. The European Surveillance of Antimicrobial Consumption (ESAC; http://www.esac.ua.ac.be) (Citation5) project proposed a set of 21 disease-specific antibiotic prescribing quality indicators (APQI) to assess the quality of antibiotic prescribing in primary care (Citation6).
To calculate values for these indicators data is needed linking antibiotic prescriptions with patients’ age, gender and diagnosis. This kind of linked data is not readily available in contrast to the data to calculate the ESAC drug-specific quality indicators, which have been routinely collected within the ESAC project (currently managed by the European Centre for Disease Prevention and Control (ECDC) as ESAC-Net) (Citation7). Although other projects have shown that such linked data exist in most European countries (Citation8), to our knowledge no quality assessment using APQI has been published so far. In addition, we believe that a description of the methodology used to extract the necessary data and its feasibility could inspire and facilitate further implementation of these indicators.
Therefore, in this study, the aim is assessing the feasibility of calculating values for 21 APQI and assessing the quality of antibiotic prescribing in office hours and out-of-hours general practice. We hypothesize that the quality indicators in out-of-hours general practice will be worse than in office hours general practice.
METHODS
Design
Calculation of values for the 21 APQI requires databases with information on diagnosis and antibiotic prescribing. For the assessment of antibiotic prescribing in office hours and out-of-hours, we extracted data from the Intego (http://www.intego.be) and Deurne-Borgerhout (HDB) databases, respectively (details see Primary care databases). Within both databases diagnoses are labelled using the revised second edition of International Classification of Primary Care (ICPC-2-R) and antibiotic prescriptions are labelled using Anatomical Therapeutic Chemical (ATC) classification (Citation9–10).
Disease-specific antibiotic prescribing quality indicators (APQI)
For seven indications (acute bronchitis/bronchiolitis, acute upper respiratory infection, cystitis/other urinary infection, acute tonsillitis, acute/chronic sinusitis, acute otitis media, and pneumonia) labelled with ICPC-2-R codes (R78, R74, U71, R76, R75, H71 and R81, respectively; see Box 1) ESAC proposed three indicators: (a) the percentage of patients with age and/or gender limitation having been prescribed an antibiotic; (b) the percentage of patients with age and/or gender limitation having been prescribed an antibiotic, and receiving the guideline recommended antibiotic; (c) the percentage of patients with age and/or gender limitation having been prescribed an antibiotic, and receiving quinolones (Citation6). For each of the 21 APQI, a range of acceptable use was proposed (see data analysis).
R78: patients aged between 18 and 75 years with ICPC-2-Ra code for acute bronchitis/bronchiolitis;
R74: patients older than one year with ICPC-2-Ra code for acute upper respiratory infection;
U71: female patients older than 18 years with ICPC-2-Ra code for cystitis/other urinary infection;
R76: patients older than one year with ICPC-2-Ra code for acute tonsillitis;
R75: patients older than 18 years with ICPC-2-Ra code for acute/chronic sinusitis;
H71: patients older than two years with ICPC-2-Ra code for acute otitis media/myringitis;
R81: patients aged between 18 and 65 years with ICPC-2-Ra code for pneumonia;
aThe revised second edition of International Classification of Primary Care.
Primary care databases
During office hours: Intego database. Fifty-four practices including 94 GPs spread all over Flanders, participate in this morbidity registration network, which is financed by the Flemish Agency for Care and Health and managed by the Academic Centre for General Practice of the Catholic University of Leuven. Patient identification information is coded in each general practice using a one-way algorithm before sending the data to the central database in Leuven. A trusted third party codes the practice and patient codes. As a result, only the registering GP is able to find out which patient matches a certain code. According to the national privacy law, patients are informed about the ongoing registration through a poster on the wall in the waiting room of the registering GP. A programme-specific code is used to code the diagnosis, which can then be converted to ICPC-2-R code. Medications are classified using their national code (CNK-code), which can be converted to an ATC–code.
The Intego procedures were approved by the ethical review board of the Medical School of the Catholic University of Leuven under No. ML1723.
Out-of-hours service centre Deurne-Borgerhout (HDB). The HDB is open only during weekends and public holidays. All GPs (n = 155, between 27 June 2003 and 30 June 2013) that work in HDB have to use the software that was specifically created for the out-of-hours service centre i.e. HWP Mailer®. Diagnoses are selected using Thesaurus terms in 3BT, the Belgian Bilingual Biclassified Thesaurus (Citation11), which is linked to ICPC-2-R. By selecting a diagnosis in 3BT, an ICPC – 2 -R code and an ICD – 10 code are automatically linked.
Prescriptions are registered using the database of the Belgian Centre for Pharmacotherapeutic Information (BCFI; http://www.bcfi.be), which is linked to the ATC classification system.
An administrator (Ph. R.) reviews all the reports to control the validity of the data. The administrator can add, alter or remove the diagnosis or prescription, e.g. to code information found in the free text.
The procedures were approved by the Committee for Medical Ethics of the University hospital of Antwerp under No. 12/49/404.
Methodology used to extract the necessary data
In both databases, we extracted antibiotic prescription data for each new diagnosis of acute bronchitis/ bronchiolitis (R78), acute upper respiratory infection (R74), cystitis/other urinary infection (U71), acute tonsillitis (R76), acute/chronic sinusitis (R75), acute otitis media (H71) and pneumonia (R81).
For the Intego database, only initial visits, not repeat visits or follow-up visits were included in the analysis, as in principle Intego GPs do not register repeat visits or follow-up visits as new episodes, unless a new diagnosis is registered. For the HDB database, the concepts of ‘repeat visits’ and ‘follow-up visits’ are not applicable.
Our unit of analysis was illness episodes, so each patient could have several episodes of the same illness. For each episode, we determined whether an antibiotic was prescribed or not when presenting for the first time. Prescription of medication is not directly linked to diagnosis. Diagnoses and prescriptions were linked when recorded on the same date. Those receiving more than one package of antibiotics on the same date were only counted once for the percentage of patients prescribed an antibiotic (indicator a). Patients with a relevant diagnosis and an antibiotic prescription on the same date were included to assess the percentage prescribed the recommended antibiotic (indicator b) and the percentage prescribed quinolones (indicator c). Those receiving both the recommended antibiotic and a quinolone at the same time were counted once for indicator b and once for indicator c.
Data analysis
Feasibility of applying the APQI. We assessed the number of APQI for which values could be calculated.
Outcome of the quality assessment. We compared the APQI values of the Intego and HDB database with the proposed ranges of acceptable use (Citation6). The acceptable range of the percentage of patients prescribed an antibiotic (indicator a) ranges from 0–20% for acute upper respiratory tract infection, tonsillitis, sinusitis and otitis media; from 0–30% for bronchitis; from 80–100% for cystitis and from 90–100% for pneumonia. A percentage of 80–100% is considered the acceptable range for the percentage of those prescribed the recommended antibiotic (indicator b). The acceptable range for the percentage of those prescribed quinolones (indicator c) ranges from 0–5%. Data was analysed using Microsoft Excel 2010®.
RESULTS
Feasibility of applying the APQI
Both databases allow calculation of all 21 APQI values ( and ).
Figure 1. Disease-specific quality indicators for antibiotic prescribing in Flemish general practice during office hours (2003–2010). (a) The percentage of patients prescribed an antibiotic (indicator a). R78, R74, U71,R76, R75, H71, R81: see Box 1. (b) The percentage of patients prescribed an antibiotic, and receiving the antibiotic recommended by the guideline (indicator b). (c) The percentage of patients prescribed an antibiotic, and receiving a quinolone (indicator c).
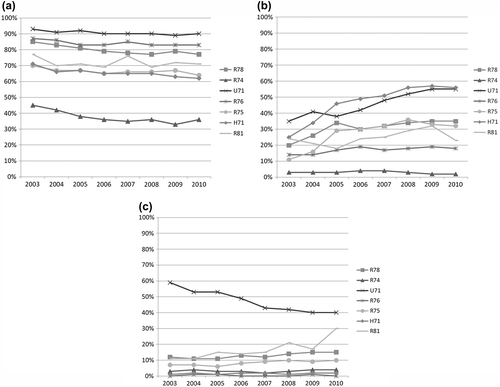
Table 1. Average value (%) for the disease-specific antibiotic prescribing quality indicators over a six-year period (from 1 January 2004 to 31 December 2009) during office hours and out-of-hours.
Outcome of the quality assessment
Antibiotic prescription during office hours ().
Patients prescribed an antibiotic (indicator a). Between 2003 and 2010, the percentage of patients prescribed an antibiotic (indicator a) was decreasing for all indications () suggesting an overall reduction in the number of antibiotic prescriptions per active patient. The relative decrease was highest for upper respiratory tract infections (–20%). In 2010, urinary tract infection (U71; cystitis/other urinary infection) was the only condition in which the percentage of patients prescribed an antibiotic (indicator a) was within the proposed range of acceptable use. Prescribing for pneumonia and upper respiratory tract infections deviated from the target by less than 20%, while bronchitis, sinusitis and otitis media missed the target by more than 40%. Prescribing for tonsillitis deviated the most, with a 63% difference.
Percentage prescribed the recommended antibiotic (indicator b). We found an increasing trend over the period 2003–2010, with an average annual increase of more than 5% for bronchitis, cystitis, sinusitis and otitis media (). However, even by 2010, the use of recommended antibiotics was not within the proposed range of acceptable use for any of the conditions under study.
Percentage prescribed quinolones (indicator c). Use of quinolones exceeded the 5% upper limit of acceptable use for four conditions (acute bronchitis, sinusitis, pneumonia, acute cystitis (). In 2010, cystitis and pneumonia deviated the most, by 40% and 30%, respectively. For cystitis, the trend was towards more acceptable use of quinolones (–32%) but was still distant from the acceptable range of 0–5%. For bronchitis and sinusitis, a slow but steady increase was observed, while for pneumonia this increase was steeper (+19%).
Out-of-hours compared to office hours ()
The analysis of the HDB database was based on 49 117 reports registered by 125 GPs between 1 January 2004 and 31 December 2009. A diagnosis was registered in 48 816 reports (99.39%). In 10 406 reports (21.19% of total), the diagnostic code was added or corrected by the administrator based on information found in the free text field.
compares the average value of the 21 quality indicators between the two available datasets over a six-year period (2004–2009). The percentage of patients prescribed an antibiotic (indicators a) and the percentage of those prescribed the recommended antibiotic (indicators b) fall within the same range in both settings. Quinolone use is lower in the out-of-hours setting for acute bronchitis, sinusitis and pneumonia, but the difference is small.
DISCUSSION
Main findings
Calculation of all 21 APQI was feasible in both databases despite their different structure.
In regard to the quality of antibiotic prescribing in Flanders, cystitis was the only condition in which the percentage of patients prescribed an antibiotic (indicator a) was within the proposed range of acceptable use. The proportion of prescribed antibiotics that were recommended antibiotics (indicator b) was below the acceptable range (80–100%) for all indications. The percentage of quinolones (indicator c) was within the acceptable range (0–5%) for otitis media, upper respiratory infection and tonsillitis. For acute bronchitis and pneumonia, none of the three indicator values fell within the proposed range of acceptable use. The similar results in both databases suggest that a similar patient population and illness severity is presenting during office hours and out-of-hours in Flanders (Belgium).
Strengths and limitations
The proposed APQI we used have face and content validity. They were internationally validated by an expert panel from 24 different countries all over Europe and Israel. Since all quality indicators are well-defined, the risk of misinterpretation is limited. In addition, for all indicators an evidence base was provided based on current national guidelines and this was scored as relevant (Citation6).
Some of the variations revealed by routine data may reflect real and important variations in actual health care quality, i.e. inappropriate antibiotic use, that merit further investigation and action, but some apparent variation may also arise because of other misleading factors such as unadjusted case mix differences (Citation12).
In this study, we were not able to distinguish initial visits from ‘repeat visits’ (initiated by the patient) or ‘follow-up visits’ (scheduled by the doctor) both in the HDB and the Intego database. Yet, in both databases we have looked at antibiotic prescribing in each case where a new diagnosis was made for the seven APQI indications. Hence, we consider these databases to be comparable. Excluding of other than first time presentations in the HDB dataset, would not alter the conclusion that indicator values are not worse in the out-of-hours primary care compared to office hours primary care.
Using information from recorded diagnoses can introduce bias due to incomplete or incorrect registration of diagnostic codes (Citation13). When there is information bias in the documentation of diagnoses in relation to the treatment status, the use of diagnostic codes alone can mislead both policy makers and health care providers about the performance scores of quality indicators. In such cases, a combination of diagnostic codes and clinical information is recommended for prescribing quality assessment (Citation14). If register-based proxies for diagnoses, disease severity or risk factors are employed, validation is essential (Citation15). Assessment of the concurrent validity of these indicators by comparing to a 'gold standard’ quality assessment at the patient level using all available clinical information will be an important next step. Such a thorough quality assessment was performed for acute otitis media in Dutch general practice using the Dutch national guideline as the gold standard (Citation16).
Some limitations have to be taken into account when considering the use of these APQI (Citation6). Indicators expressing the percentage of patients prescribed an antibiotic (indicator a)—where lower scores represent better quality of care except for cystitis and pneumonia—might be influenced by different thresholds for consulting a GP. For example, in a practice with a high threshold to consult, only patients with severe symptoms might visit the GP and this could result in a higher prescribing rate compared to practices with a lower threshold. However, we believe this limitation is probably more relevant when APQI values are compared between countries, and not within a country as it is reasonable to expect similar consulting thresholds in practices within the same country. Delayed prescribing is another reason for potential bias in the interpretation of values for these quality indicators. Furthermore, when a patient is referred for second line treatment, the treatment will not be initiated by the GP and, therefore, it will not be included in the database. This explains the relative low use of antibiotics for pneumonia (indicator 7a) compared to that for bronchitis (indicator 1a). Note reviews of the HDB database showed that patients with pneumonia who did not receive antibiotics were referred to the emergency department. The percentage of patients prescribed the recommended antibiotics (indicator b)—where higher scores represent better quality of care—can be biased by country specific guidelines recommending other antibiotic classes as first line therapy. However, in this study, Belgian recommendations for anti-infectious treatment in ambulatory care (Citation17) and the APQI are entirely in line. In general, the percentage of patients prescribed quinolones (indicator c)— where lower scores represent better quality of care—can be biased by specific resistance patterns. Yet, in Belgium, however, resistance of respiratory and urinary pathogens to recommended antibiotics is not high (Citation18).
Interpretation in relation to existing literature
Cystitis was the only condition in which the percentage of patients prescribed an antibiotic (indicator a) reached the target. Fewer patients than acceptable received an antibiotic for pneumonia, while for most conditions too many antibiotics were prescribed. National antibiotic awareness campaigns have been run in Belgium since 2000. Although there is no hard scientific evidence for a cause–effect relationship, Belgium experienced a 36% reduction in antibiotic prescribing between the winter season 2000–2001 and 2006–2007 (Citation19,Citation20). This downward trend can be observed in indicator (a) but is less pronounced. This might be because we used active patients as our denominator instead of inhabitants. In addition, the study population is limited to general practitioners (versus all prescribers in ambulatory care) and to Flanders (versus Belgium).
Despite these awareness campaigns, antibiotic prescribing rates in Belgium are still unfavourable compared to many other European countries (Citation21). Furthermore, the proportion of macrolide-resistant Streptococcus pneumoniae isolates and the proportion of fluoroquinolone-resistant Escherichia coli isolates are still higher than in lower prescribing countries (Citation18).
A striking observation is the low use of recommended antibiotics in general practice. For most conditions, less than half of the prescribed antibiotics are the recommended ones. A positive trend over time for all indications except upper respiratory tract infections is encouraging, but the proportions in 2010 (the final year of our study) were still considerably below those proposed as the acceptable range.
Since these quality indicators are based on international consensus of guideline recommendations, one could argue whether they are all applicable to Belgium (Flanders). The availability of beta-lactamase sensitive penicillins on the pharmaceutical market in Belgium has not been continuous (e.g. withdrawal of phenoxymethylpenicillin in 1997, re-introduction in 1999, withdrawal of clometocillin in 2011), which could cause reluctance to prescribe these substances.
Nevertheless, we believe our observations offer a huge opportunity for quality improvement in Flemish general practice. Further campaigns should not only focus on prescribing fewer antibiotics, but also target the choice of antibiotics (Citation21,Citation22).
These interventions could also help to reduce quinolone use for lower respiratory tract infections and cystitis. In particular, the progressively increasing use of quinolones to treat lower respiratory tract infections could be considered as an alarming observation (Citation23). As quinolone use should be restricted and mainly reserved for well-defined indications, such high use suggests non-adherence to prescribing guidelines.
Implications for clinical practice and policy
This study illustrates that the APQI are easy to implement using databases linking diagnosis and prescription data. We suggest using the APQI at the level of the individual primary care prescriber using electronic medical records with a link to ICPC coding for diagnosis, and a link to ATC coding for antibiotic prescriptions. It is hoped the publication of this study will result in increased accessibility of these routinely collected data sources and could trigger Electronic Health Record (EHR) software vendors to provide an EHR-tool, which allows GPs to calculate their own APQI values. Data for the ESAC quality indicators is easy to collect, unlike other primary care quality indicators that require EHR information like CRP values or Anthonisen criteria (Citation24).
In addition, implementation of the disease-specific indicators would improve representativeness and could allow policy makers to analyse thoroughly antibiotic prescribing in primary care in their respective countries.
Implications for research
Assessment of the concurrent validity of these indicators by comparing to a 'gold standard’ quality assessment at the patient level using all available clinical information will be an important next step.
We suggest the development of an EHR-tool to generate APQI values and to analyse if implementation of this tool would improve the antibiotic prescribing quality of the individual GP.
Conclusion
Application of APQI is feasible for use in databases linking diagnosis and prescription data. Assessment of APQI revealed suboptimal quality of antibiotic prescribing in Flemish general practice, both during and outside office hours. In particular, the use of recommended antibiotics offers a huge opportunity for quality improvement.
ACKNOWLEDGEMENTS
The authors should like to thank Sibyl Anthierens and Nick Francis for their suggestions.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet. 2005;365:579–87.
- Malhotra-Kumar S, Lammens C, Coenen S, Van Herck K, Goossens H. Impact of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci among healthy volunteers: A randomised, double-blind, placebo-controlled study. Lancet. 2007;369:482–90.
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. Br Med J. 2010;340:c2096.
- Ashworth M, Charlton J, Ballard K, Latinovic R, Gulliford M. Variations in antibiotic prescribing and consultation rates for acute respiratory infection in UK general practices 1995–2000. Br J Gen Pract. 2005:603–8.
- Coenen S, ESAC Management Team and the ESAC Project Group. European surveillance of antimicrobial consumption (ESAC). Eur J Gen Pract. 2005;11:42.
- Adriaenssens N, Coenen S, Tonkin-Crine S, Verheij TJM, Little P, Goossens H. European Surveillance of Antimicrobial Consumption (ESAC): Disease-specific quality indicators for outpatient antibiotic prescribing. BMJ Qual Saf. 2011;20:764–72.
- Coenen S, Ferech M, Haaijer-Ruskamp FM, Butler CC, Vander Stichele RH, Verheij TJM, et al. European Surveillance of Antimicrobial Consumption (ESAC): quality indicators for outpatient antibiotic use in Europe. Qual Saf Health Care. 2007; 16:440–5.
- Fleming D. Track E10: Workshop: The potential of electronic medical records for health service management. Eur J Public Health. 2006;16:104.
- Lamberts H, Wood M. ICPC. International classification of primary care. Oxford: Oxford University Press; 1987.
- WHO Collaborating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical (ATC) classification system: Guidelines for ATC classification and DDD assignment 2011. Norwegian Institute of Public Health. Oslo . Available at http://www.whocc.no/filearchive/publications/2011guidelines.pdf (accessed 8 July 2013).
- The creation of the Belgian Bilingual Bi-encoded Thesaurus (3BT) . Available at http://www.semantichealth.org/PUBLIC/Belgium_The%20creation%20of%203BT.pdf (accessed 8 July 2013).
- Powell AE, Davies HTO, Thomson RG. Using routine comparative data to assess the quality of health care: Understanding and avoiding common pitfalls. Qual Saf Health Care. 2003;12: 122–8.
- Botsis T, Bassoe C, Hartvigsen G. Sixteen years of ICPC use in Norwegian primary care: Looking through the facts. BMC Med Inform Decis. 2010;10:11.
- Martirosyan L, Arah OA, Haaijer-Ruskamp FM, Braspenning J, Denig P. Methods to identify the target population: Implications for prescribing quality indicators. BMC Health Serv Res. 2010; 10:137.
- Andersen M. Is it possible to measure prescribing quality using only prescription data?Basic Clin Pharmacol. 2006;98: 314–9.
- Akkerman AE, Kuyvenhoven MM, van der Wouden JC, Verheij TJM. Analysis of under- and overprescribing of antibiotics in acute otitis media in general practice. J Antimicrob Chemother. 2005;56:569–74.
- Belgian Antibiotic Policy Coordination Committee (BAPCOC). Belgian guide for anti-infectious treatment in ambulatory care (in Dutch). Federal Public Service Health, Food Chain Safety and Environment . Brussels, Belguim; 2008. Available at http://www.health.belgium.be/filestore/15616531/832250_BW_NL_01_84_IC_156 16531_nl.pdf (accessed 8 July 2013).
- European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2011. Annual report of the european antimicrobial resistance surveillance network (EARS-Net). Stockholm: ECDC; 2012.
- Goossens H, Coenen S, Costers M, De Corte S, De Sutter A, Gordts B, et al. Achievements of the Belgian Antibiotic Policy Coordination Committee (BAPCOC). Euro Surveill. 2008;13: 10–3.
- Huttner B, Goossens H, Verheij T, Harbarth S, on behalf of the CHAMP consortium. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis. 2010;10:17–31.
- Adriaenssens N, Coenen S, Versporten A, Muller A, Minalu G, Faes C, et al. European surveillance of Antimicrobial Consumption: Outpatient antibiotic use in Europe (1997–2009). J Antimicrob Chemother. 2011;66:vi3–12.
- Coenen S. Infectious diseases in primary care; managing the interface between the person and the community. Eur J Gen Pract. 2012;18:117–21.
- Adriaenssens N, Coenen S, Versporten A, Muller A, Minalu G, Faes C, et al. European surveillance of Antimicrobial Consumption: Outpatient quinolone use in Europe (1997–2009). J Antimicrob Chemother. 2011;66:vi47–56.
- Hansen MP, Bjerrum L, Gahrn-Hansen B, Jarbol DE. Quality indicators for diagnosis and treatment of respiratory tract infections in general practice: A modified Delphi study. Scan J Prim Health. 2010;28:4–11.

