ABSTRACT
Background: The systems-based management of laboratory test ordering and results handling is a known source of error in primary care settings worldwide. The consequences are wide-ranging for patients (e.g. avoidable harm or poor care experience), general practitioners (e.g. delayed clinical decision making and potential medico-legal implications) and the primary care organization (e.g. increased allocation of resources to problem-solve and dealing with complaints). Guidance is required to assist care teams to minimize associated risks and improve patient safety.
Objective: To identify, develop and build expert consensus on ‘good practice’ guidance statements to inform the implementation of safe systems for ordering laboratory tests and managing results in European primary care settings.
Methods: Mixed methods studies were undertaken in the UK and Ireland, and the findings were triangulated to develop ‘good practice’ statements. Expert consensus was then sought on the findings at the wider European level via a Delphi group meeting during 2013.
Results: We based consensus on 10 safety domains and developed 77 related ‘good practice’ statements (≥ 80% agreement levels) judged to be essential to creating safety and minimizing risks in laboratory test ordering and subsequent results handling systems in international primary care.
Conclusion: Guidance was developed for improving patient safety in this important area of primary care practice. We need to consider how this guidance can be made accessible to frontline care teams, utilized by clinical educators and improvement advisers, implemented by decision makers and evaluated to determine acceptability, feasibility and impacts on patient safety.
Laboratory test ordering and results management are a source of error and avoidable harm in primary care settings worldwide
The LINNAEUS collaboration developed good practice guidance to assist primary care teams in reviewing, developing and implementing safe systems and processes for ordering tests and managing results.
INTRODUCTION
The reliability of formal ordering and tracking systems to oversee the management of laboratory test requests and subsequent results handling is problematic and a known source of error in primary care settings worldwide (Citation1–4). For patients and their relatives this may have multiple consequences in terms of contributing to avoidable harm and unnecessary distress, sub-optimal clinical management of illness and delayed treatments (Box 1), poor experience with care, miscommunication of test results by healthcare staff, and the inconvenience of return appointments, repeating blood tests or making formal complaints (Citation5–7). However, the absence of rigorous research in this area means it is difficult to reliably quantify the scale and extent of the issue.
A 68-year old patient attended the family doctor complaining of non-specific tiredness. The doctor ordered a thyroid check to exclude hypothyroidism, a glucose level to exclude diabetes and a full blood count (FBC) to exclude anaemia. The patient's blood sample was taken two days later by the phlebotomist. The patient phoned one week later for the results. The administrator informed the patient that the doctor said that the results were ‘normal.’ The administrator did not realize that although the thyroid and sugar test were normal, the FBC had not been returned to the practice. When the patient attended a further appointment, three-months later, it was noticed that the FBC had not been returned to the practice. The original result had been sent to the wrong practice. It demonstrated a possible iron deficiency anaemia. Follow-up investigations confirmed the iron deficiency with secondary care investigation finding the cause was a colonic cancer.
For general practitioners (GPs), a range of professional, legal and accountability issues make the safe handling of laboratory test results a high-level priority because of the patient safety evidence outlined (Box 2). Poor laboratory test follow-up, missed results and delays in communication are likely to have a greater impact on patients whose investigation outcomes point to the need for timely medical treatment or intervention. Lack of, or inadequate, results handling systems can therefore lead to clinical judgments on diagnostic and treatment decisions being delayed based on the availability of incomplete information, which limits therapeutic options thereby potentially impacting on the safety of patient care (Citation5–6,Citation8–10).
Laboratory test ordering and results handling processes are a source of error and avoidable patient harm in primary care.
Lack of, or inadequate, safety systems to guide ‘good practice’ and mitigate errors are common, creating risks for patients and GPs.
Safety is created, and risks minimized by introducing and standardizing processes to improve the reliability of results management systems.
However, the practice culture must embrace a systems approach to this issue and a commitment to staff training and development.
The outputs of this development work are of direct interest to frontline clinicians, managers and staff, educators, patient safety advisors, health service researchers, professional bodies, and policymakers.
The implications for the GP of this type of unsafe practice may also include having to cope with formal complaints by patients or relatives, litigation claims for financial compensation in the event of proven clinical negligence or upheld complaints, and possible licensure sanctions by medical regulators (Citation1–6). A further consequence may be a difficulty in repairing and maintaining the bond of trust in the doctor–patient relationship (Citation5,Citation7,Citation11,Citation12).
At the system level, the published evidence suggests that general practices do not always have adequate processes to track requests for investigations, to record the results of clinically significant abnormal investigations that are returned from laboratories, or to confirm whether follow-up action has taken place before results reports are filed in patients’ records (Citation6,Citation8–10). Furthermore, in many countries general practices do not have effective systems in place to reliably notify patients of normal results either, thereby shifting a level of responsibility onto the patient to contact the practice to obtain the results (Citation5,Citation9). This is despite medico-legal accountability for tests ordered resting with the requesting clinician (Citation11). The impact of poor results handling is evident in the associated financial costs incurred and use of human resources to problem-solve system failures and repeat related work tasks (Citation8). Additionally the experience of poor healthcare service by patients may also have wider local community impacts in terms of creating bad publicity or a poor professional reputation (Citation5,Citation13).
In the absence of specific guidance on this important topic, we aimed to take a rigorous research-based approach to identifying, developing and building consensus on ‘good practice’ guidance statements to inform the frontline implementation of safe systems for ordering laboratory tests and managing results in primary care settings internationally.
METHODS
Scope of Study
Internationally there is variation in primary healthcare design, provision of information technology decision support and practice systems for coordination of laboratory test ordering and result handling in and across countries. A key study focus was to ensure that the ‘good practice’ guidance generated was largely relevant in principle to all countries, regardless of the primary care systems in operation. Also, given the scope, range and complexity of clinical investigations that can be ordered by primary care clinicians, the authors decided to narrow the study focus to include only common, high volume biochemistry and haematology blood test requests—although it was recognized that the principles underpinning any developed guidance would apply more widely.
Study design
We undertook a mixed methods qualitative study in the UK and Ireland contexts and then sought consensus on our findings at the wider European level between September 2011 and April 2013 (). As a first step, we formed a steering group to co-ordinate project networking and development activities, and then analyse, interpret and integrate study result data as they were generated. The group consisted of highly experienced frontline UK and Ireland primary care staff representatives including GPs, practice nurses and practice managers, as well as patient safety researchers, clinical educators, and human factors and medical indemnity specialists (four authors were also group members—PB, EF, JP and JM).
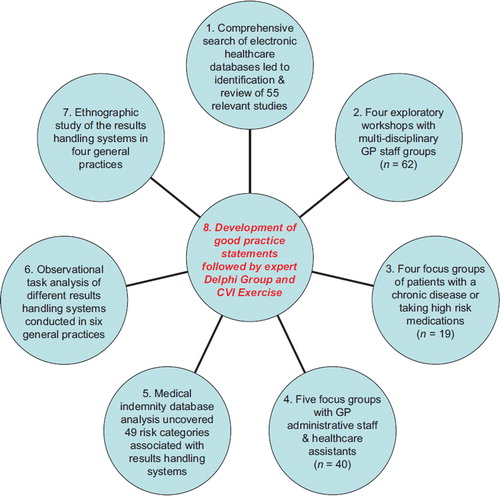
Figure 1. Mixed study methods applied to generate ‘good practice’ statements and achieve expert consensus using a Delphi group and content validity index (CVI) exercise. The timescale of most studies overlapped rather than being undertaken in sequential order. Studies 1, 2, 3, 4, 6 and 8 were led by NHS Education for Scotland. Study 5 was led by the Medical Protection Society. Study 7 was led by the University of Dundee. Study 8 was led by NHS Education for Scotland and involved all other research partners.
Data collection and analyses
As the qualitative data from each of the seven contributory studies briefly outlined in were generated, they were integrated and themed in line with what appears to be the only published generic system framework for laboratory test requests and results handling (Citation12). The process began with three authors (PB, EF and JM) independently reading and re-reading the information generated by each contributory study (e.g. a written report on the findings of focus groups undertaken or a content analysis of the qualitative content of a medical indemnity database). Each then began to theme these findings and link relevant issues (e.g. the importance of a tracking process for reconciling tests orders with results received) to the most appropriate stages of the aforementioned framework by recording these on a blank pro forma aligned to each stage.
Next, based on repeated comparisons of the written contents of the completed pro forma, a series of linked ‘good practice’ statements were then jointly developed, refined, merged, or deleted on an iterative basis by the three authors during six three-hour sessions of small group work (and follow-up email correspondence) to gain agreement on the relevance and wording of each statement and its relevance to each framework stage. The lead author chaired these sessions and wrote down the agreed statement versions—that were then checked and verified via email after each session by JM and EF. All statements included were triangulated (Citation14) with emergent findings, i.e. to be included as a statement, the issue at hand had to have been raised as a safety concern in a minimum of three of the seven contributory studies (which was feasible because all focused explicitly on understanding safety issues of interest to the study). Where appropriate, we also divided the developed statements into ‘communication,’ ‘process’ and ‘systems’ sub-categories to aid relevance and usability.
The statements were then presented to the steering group for reflection, critical appraisal and feedback over the course of three separate full-day meetings and by email, until consensus was reached on each theme and the inclusion of related ‘good practice’ statements or otherwise. The main output of this activity was a draft document divided into 10 safety domains with a total of 93 related statements of ‘good practice’ identified and agreed. For pragmatic reasons of time and available funding, no external validation of the quality of data analysis was performed.
Final consensus building
From within the LINNEAUS collaboration, we emailed all participating project leads from each country to participate voluntarily in the consensus-building element of the study. Those interested were sent the draft document for feedback on salient language, cultural and system issues that might help us to improve comprehension of the written statements within their national contexts. On our behalf, these project leads then identified and invited one or two clinicians from their country—who were judged to have relevant expert knowledge of the subject area—to attend a Delphi Group (Citation15) meeting in London in March 2013. A content validity index (CVI) exercise was also used in conjunction with the Delphi method to help quantify a final cut-off point for statement inclusion in the guidance (Citation16).
A total of 10 international experts representing 11 discrete national healthcare systems for handling laboratory results attended the London meeting. One professional worked in five different national primary care systems in the UK and all-Ireland, which gave them a unique cross-cutting perspective. Other participants represented Scotland (n = 2), France (n = 1), Greece (n = 1), Poland (n = 2), Spain (n = 2) and the Netherlands (n = 1).
The purpose was to critically appraise and ultimately agree on a final set of high-level safety domains and related ‘good practice’ statements of relevance in international settings (irrespective of whether some or many elements of this guidance were currently desirable or achievable within the differing political, economic, or healthcare policy contexts with regard to improving patient safety in primary care). Experts were also asked to identify any missing issues of high relevance that they deemed important for guidance inclusion.
RESULTS
The final international meeting led to consensus on 10 generic safety domains and the inclusion of 77 ‘good practice’ statements that were judged by experts to be relevant to creating patient safety and minimizing risks in laboratory test ordering and subsequent results handling systems in European primary care settings ().
Table 1. Ten high-level safety domains and examples of ‘good practice’ statements. The complete set of good practice statements is provided as a web-only file.
Application of the CVI exercise led to full agreement by the expert group on retaining the 10 safety domains identified in the initial consensus building development work undertaken in the UK and Ireland contexts. However, 16 of the previously developed 93 ‘good practice’ statements failed to achieve the necessary minimum 80% agreement levels amongst the expert group and these were omitted from the final guidance. There was much discussion on the possibility of developing other safety domains or new statements of ‘good practice’, but after consideration, no new additions were appended.
DISCUSSION
Main findings
We developed expert consensus on safe laboratory test ordering and results management systems in selected European primary care settings. Of the 10 safety domains identified, the first two are concerned with the requirements needed to engender and maintain a commitment to improving local safety culture amongst team members, and the necessity for training and development of staff in relation to results handling. Both domains recommend taking a whole systems approach as one way to improve primary care team knowledge and understanding of the different human interfaces, interactions, responsibilities and actions that are necessary to build and maintain system reliability (Citation6,Citation12,Citation17,Citation18).
The remaining eight domains focus directly on outlining the guiding principles identified as creating safety at each discrete stage of the laboratory test ordering and results handling system. Specifically, much of the detail in these statements underpins the need for practice teams to ensure that they have a recognized high-reliability process for tracking clinical investigations ordered by clinicians, reconciling these ordered tests with results received from the laboratory, ensuring that the results are then clinically reviewed and actioned or filed, and the outcome is safely communicated to the patient with follow-up arranged, where clinically appropriate (Citation6–13,Citation17,Citation18).
Strengths and limitations
A key strength was the application of a diverse range of qualitative research methods as a means of identifying error prone human-system interaction issues and capturing ‘good practice.’ This allowed us to triangulate findings from different data sources. We were also able to recruit sufficient numbers of experienced primary care team members to assist in the development work, giving them space and time to think through the safety-critical problems affecting their practice systems. Two methods where particularly innovative and of value in studying the topic: undertaking observational task analyses (Citation23) of results management systems in a range of practices; and interrogating a national medical indemnity database containing information on a plethora of risks associated with results management systems—enabling us to tap into arguably the largest known source of empirical data on the subject.
The main limitations were the over-emphasis on consensus development in the UK and Ireland contexts and the relatively small numbers of participants—particularly at the European level, where we were unable to recruit experts from several countries (Denmark, Germany, and Austria)—meaning that the developed consensus may be biased and not truly representative of the relevant safety issues affecting international results management systems. For example, the guidance reflects the situation in the UK and some other countries where it is normal (because of heavy workload issues) for administrative staff to communicate most test results to patients, whereas in other European countries this task is performed by a clinician. Further research and consultation is, therefore, necessary to bridge this gap, particularly in relation to the lack of epidemiological data on the scale and nature of related risks. However, there was a high level of concordance with the UK and Ireland issues raised in both the published literature (Citation6,Citation8–12,Citation18,Citation19) and also when developing consensus with the international experts, who strongly agreed that the safety principles underpinning the great majority of ‘good practice’ statements translated to their national contexts. The study scope and findings focus on high volume blood test requests, which may not be generalizable to other test ordering systems such as for radiology investigations, or less frequently ordered blood test requests. Finally, the volunteer stakeholders involved may not be representative of those with the greatest knowledge of the safety issues, while alternative research methods may have offered more penetrating and broader insights.
Implications for primary care teams
At a fundamental level, primary care teams can immediately adapt elements of this guidance to help them develop new systems or augment existing systems, to minimize the risk of error and avoidable harm to patients. Similarly, it may also be used to improve existing formal introduction packs for new GPs, temporary doctors, GP trainees and other staff groups to educate them about the associated safety implications and professional accountability expectations, as well as describing how the internal system is designed and the related roles and responsibilities of relevant team members. In particular, for individual GPs, the guidance reinforces the fact that the ultimate responsibility for following-up blood test results lies with the clinician who ordered the test, therefore relying (as many do) on the patient to contact the practice is considered a potential clinical and medico-legal risk (Citation7,Citation11).
Unlike other health sectors (e.g. acute hospital care), primary care teams in many countries inhabit comparatively small non-bureaucratic organizations that have the power and resources to develop and re-design their own internal systems for managing patient care, including laboratory results handling. The GP, and to a lesser extent the practice manager and the administrative support staff, is pivotal to the decision-making and problem-solving governing this aspect of care. However, a key issue for GPs in considering the safety improvement merits of this guidance is to appreciate more explicitly the psycho-social factors (e.g. low levels of work autonomy, poor working relationships, heavy workloads and high job stress amongst individual staff members) and socio-cultural and organizational issues (e.g. staffing levels, strength of team working, commitment to staff training and professional development and quality improvement, and dominant power hierarchies) that will influence the effective implementation of guidance (Citation19–20). Therefore, where existing psychosocial and practice culture issues militate against this purely ‘technical solution’, then it is likely that problems will arise when guidance implementation is attempted (Citation21).
For primary care educators, the implications of this work may include the need for short targeted training interventions for GPs and other frontline staff. For example, on how GPs could communicate test results in more specific, precise and fewer ambiguous terms to frontline administrators to enable them in turn to inform patients in a safe, effective manner and with greater clarity. Similar interventions are also needed for key staff on improving knowledge of whole systems thinking in the workplace (Citation22), and perhaps even on interpreting and implementing aspects of the guidance itself.
It is likely that much work is required to raise awareness of both the safety-critical nature of the results handling issue and the developed guidance amongst policy makers, senior healthcare management, clinical leaders and professional bodies across most European primary care systems. Potential indicators of success in this regard would be inclusion of the topic in national patient safety programmes or through pay-for-performance or quality accreditation schemes, while allocation of funding for related quality improvement or research initiatives may also be a favourable outcome.
Conclusion
We have identified and prioritized a whole range of safety-critical issues judged to be relevant to ‘good practice’ in the systems-based management of laboratory results by frontline primary care team members, international experts and safety specialists. It is likely that a method for measuring compliance with the ‘good practice’ principles developed will be necessary to assess system reliability and provide primary care teams with feedback to direct ongoing safety improvement efforts in this important area.
Supplementary material available online
Supplementary Appendix available at http://dx.doi.org/10.3109/13814788.2015.1043724
igen_a_1043724_sm2826.pdf
Download PDF (39.1 KB)ACKNOWLEDGEMENTS
The authors offer sincere thanks to those UK general practices that allowed us to conduct observational task analyses on their systems as well as the clinicians and staff who attended and contributed to the workshops to identify good practices. The authors also thank the following individuals who made important contributions to clarifying issues and informing guidance development: Neil Hepworth, Dr Andrew Martin, Jill Gillies, Marion McLeod, Susan Kennedy, Dr Carl de Wet, Dr Hannah Hesslegreaves, Kirsteen Coady, Sheilagh MacFarlane, Dr Julie Ferguson, Susane Hogarth, Joanne Irwin, Ruth Aird, and Charlotte Leggatt. The following international experts made invaluable contributions to refining, improving and validating the guidance: Julie Price (UK & Ireland), Dr Duncan McNab (Scotland), Professor Wim Rutten (The Netherlands), Professor Miroslawa Pietruczuk (Poland), Dr Makandjou Ola Wabi Eusebio (Poland), Dr Evangelos Drosos (Greece), Dr Angels Vilanova Navarro (Spain), Jesus Sabcho Vilellas (Spain) and Professor Jean Brami (France).
FUNDING
The research leading to these results has received funding from the European Community’s Seventh Framework Programme FP7/2008–2012 under grant agreement no. 223424.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- McKay J, Bradley N, Lough M, Bowie P. A review of significant events analysed in general medical practice: Implications for the quality and safety of patient care. BMC Fam Pract. 2009, 10:61.
- Elder NC, Dovey SM. Classification of medical errors and preventable adverse events in primary care: A synthesis of the literature. J Fam Pract. 2002, 51:927–32.
- Jacobs S, O’Beirne M, Derfiingher LP, Vlach L, Rosser W, Drummond N. Errors and adverse events in family medicine: Developing and validating a Canadian taxonomy of errors. Can Fam Physician 2007, 53:271–6.
- The Health Foundation. Evidence scan: Levels of harm in primary care. January 2011. London. Available at: http://www.health.org.uk/publications/levels-of-harm-in-primary-care/ (accessed 10 May 2013).
- Makeham MA, Kidd MR, Saltman DC, Mira M, Bridges-Webb C, Cooper C, et al. The Threats to patient safety (TAPs) study: Incidence of reported errors in general practice. Med J Aust. 2006;185, 95–8.
- Elder NC, Graham D, Brandt E, Dovey S, Philips R, Ledwith J, et al. The testing process in family medicine: Problems, solutions and barriers as seen by physicians and their staff. J Patient Saf. 2005;25–32.
- Bird S. Missing test results and failure to diagnose. Aust Fam Physician 2004;33:360–1.
- Poon EG, Gandhi TK, Sequist TD, Murff HJ, Karson AS, Bates DW.‘I wish I had seen this test result earlier!’ Dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164:2223–8.
- Elder NC, McEwan TR, Flach JM, Gallimore JJ. Management of test results in family medicine offices. Ann Fam Med. 2009;7: 343–51.
- Mold JW. Management of laboratory test results in family practice: An OKPRN study. J Fam Pract. 2000;49:709–15.
- Bird S. A GP's duty to follow-up test results. Aust Fam Phys. 2003;32:45–6.
- Hickner JM, Fernald DH, Harris DM, Poon EG, Elder NC, Mold JW. Issues and initiatives in the testing process in primary care physician offices. Comm J Qual Patient Saf. 2005;31:81–9.
- Kljakovic M. Patients and tests: A study into understanding of blood tests ordered by their doctor. Aust Fam Phys. 2012;40: 241–3.
- Golafshani N. Understanding reliability and validity in qualitative research. The Qualitative Report 2003;8:597–607.
- Jones J, Hunter D. Qualitative research: consensus methods for medical and health services research. Br Med J 1995;311:76.
- Yaghmaie F: Content validity and its estimation. J Med Educ. 2003;3:23–5.
- Elder NC, McEwen TR, Flach JM, Galliemore JJ. Creating safety in the testing process in primary care offices. Agency for Healthcare Quality and Research; 2007. Available at: http://www.ahrq.gov/professionals/quality-patient-safety/patient-safety-resources/resources/advances-in-patient-safety-2/vol2/Advances-Elder_18.pdf (accessed 10 May 2013).
- Hickner J. Reducing test management errors in primary care office practice. J Patient Saf. 2005;1:70–1.
- de Wet C, Johnson P, Mash R, McConnachie A, Bowie P. Measuring perceptions of safety climate in primary care: A cross-sectional study. J Eval Clin Pract. 2010, 18:135–42.
- Apekey TA, McSorley G, Tilling M, Siriwardena AN. Room for improvement? Leadership, innovation culture and uptake of quality improvement methods in general practice. J Eval Clin Pract. 2011, 17:311–8.
- Bosk CL, Dixon-Woods M, Goeschel CA, Pronovost PJ. The art of medicine: Reality check for checklists. Lancet 2009;374:444–5.
- de Savigny D, Adam T, editors. Systems thinking for health systems strengthening. Geneva: Alliance for Health Policy and Systems Research, WHO; 2009.
- Reason J. Combating omission errors through task analysis and good reminders. Qual Saf Health Care 2002;11:40–4.
