Abstract
Congenital cataract is a leading cause of visual impairment in children and brings approximately 10% of childhood blindness worldwide. Molecular analysis revealed ~60 loci to be associated with several phenotypes of childhood cataracts. Until now, more than 30 loci and 18 genes on different chromosomes have been associated with autosomal dominant congenital cataract (ADCC). Here, we present a three-generation Italian family with a non syndromic ADCC. A linkage analysis carried out using HumanCytoSNP-12 DNA Analysis BeadChip led us to identify ten genomic regions virtually involved in the disease. All the genes located in these regions were scored for possible relationship with ADCC and, according to a strict clinical and genetic selection, 4 genes have been analyzed. A novel sequence variant was found in the CRYBB2 gene (p.Ser143Phe). This variant affects a conserved aminoacid in the third Greek key motif of the protein, cosegregates with the disease phenotype in all affected individuals and is not present both in the unaffected family members and 100 healthy control subjects. Finally, we identified the first CRYBB2 mutation in an Italian family causing a clinical picture of ADCC.
KEYWORDS::
Congenital cataract is a leading cause of visual impairment in children. Overall, its prevalence is 0.6 to 6.0 in 10 000 live births, causing approximately 10% of childhood blindness worldwideCitation1 and approximately one quarter to one third of congenital cataracts are hereditary.Citation2 Until now, more than 30 loci and 18 genes on different chromosomes have been associated with autosomal dominant congenital cataract (ADCC).Citation3 Approximately half of these mutations involve crystallins, one-quarter involve connexins, and the remaining one quarter involve the other genes.Citation4
Beta-crystallins form aggregates of different sizes and are able to self-associate to form dimers or to form heterodimers with other beta-crystallins. CRYBB2 encodes for the βB2-crystallin, the most abundant and water-soluble β-crystallin in the lens.Citation5 So far, eleven missense, one splicing and one insertion/deletion mutation have been identified in CRYBB2Citation6 (). All the families presented an autosomal dominant cataract.
TABLE 1 Mutations previously described in the CRYBB2 gene associated with congenital cataracts.
A three-generation family of Italian origin presenting with autosomal dominant congenital lamellar and bilateral cataracts was ascertained from the Department of Ophthalmology of IRCCS Burlo Garofolo Institute in Trieste, Italy. In order to make possible the sequencing of a reasonable number of genes, an exclusion linkage analysis with High Density SNP array has been performed. Then, a mutation analysis of all exons and flanking intronic sequences of the genes (EPHA2, CRYBA4, CRYBB1 and CRYBB2) in the linkage areas has been carried out.
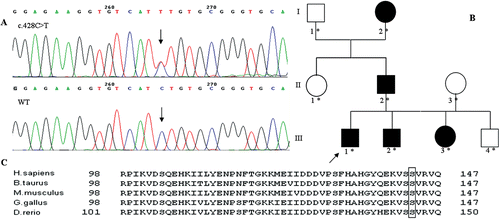
The direct sequencing of CRYBB2 gene detected a nucleotide change C to T at the position 428 of the DNA sequence (c.428C>T, ). This change leads to a novel missense mutation from a Serine to a Phenylalanine at position 143 of the protein (p.Ser143Phe ). The amino acid involved is highly conserved across species () and the consulted software predicted the mutation as damaging (Mutation Taster: 0.999; PolyPhen2: 1.000; Mutation Assessor: HIGH). The mutation was confirmed in all the affected family members by Sanger sequencing and none of the 100 healthy controls showed it. Moreover, the mutation was not present in dbSNP or 1000 genomes project. No mutations were found in the EPHA2, CRYBA4 and CRYBB1 genes.
FIGURE 1 An evolutionarily conserved Serine is mutated in our Italian family with ADCC. A. Electropherograms showing a heterozygous c.428C>T mutation in an affected individual compared to a wild type DNA. B. Schematic pedigree of the Italian family affected by CRYBB2-related ADCC. An asterisk shows the tested family members. C. Protein alignment shows conservation of the p.143th amino acid during evolution. The p.143th is highlighted.
The correct association and supramolecular assembly of lens crystallins are crucial for lens transparency and a high refractive index. In the lens, βB2-crystallin forms hetero-oligomers with other β-crystallins, an interaction that is mediated by β strands.Citation17 Besides the lens, β-crystallins are expressed in other tissues such as retina, brain, and testis implying its role in other biologic functions as well, like elongation of axons during regeneration of retinal ganglion cells.Citation18
The mutation p.Ser143Phe in our family is located in the highly conserved Serine residues in a major functional domain of the βB2-crystallin. It is the third Greek key motif of the protein, a crucial region to the correct formation of the tertiary protein structure. Given the structural studies performed on the adjacent aminoacid 146Citation9, we hypothesize that our mutation leads to the replacement of an original β-strand by an α-helix, which may affect the third Greek key motifs and change the folding properties of βB2-crystallin. The phenotype of cataract is presumed to be caused by the formation of a heavy molecular weight fraction or a decrease in the stability of βB2-crystallin. No genotype-phenotype correlation in mutations of CRYBB2 gene has been already reported.
Finally, we identified a novel missense mutation in CRYBB2 (p.Ser143Phe) associated with autosomal dominant congenital cataract in the first Italian family reported with CRYBB2 mutation. The p.Ser143Phe affects the third Greek key motif of the βB2-crystallin presumably leading to a misfolded protein. Obviously, further biophysical studies are necessary to evaluate the precise molecular mechanism caused by the mutation.
Declaration of interest: The authors declare no conflict of interest.
REFERENCES
- Reddy MA, Francis PJ, Berry V, Bhattacharya SS, Moore AT. Molecular genetic basis of inherited cataract and associated phenotypes. Surv Ophthalmol. 2004; 49 (3):300–315.
- Bermejo E, Martinez-Frias ML. Congenital eye malformations: clinical-epidemiological analysis of 1,124,654 consecutive births in Spain. Am. J. Med. Genet. 75 (1998) 497e504.
- Wang K, Wang B, Wang J, et al. A novel GJA8 mutation (p.I31T) causing autosomal dominant congenital cataract in a Chinese family. Mol Vis. 2009;15:2813–2820.
- Hejtmancik JF. Congenital cataracts and their molecular genetics. Semin Cell Dev Biol. 2008;19 (2):134–149.
- Huang B, He W. Molecular characteristics of inherited congenital cataracts. Eur J Med Genet. 2010 Nov–Dec;53 (6):347–357.
- http://www.biobase-international.com/product/hgmd
- Yao K, Li J, Jin C, Wang W, Zhu Y, Shentu X, Wang Q. Characterization of a novel mutation in the CRYBB2 gene associated with autosomal dominant congenital posterior subcapsular cataract in a Chinese family. Mol Vis. 2011 Jan 13;17:144–152.
- Santhiya ST, Kumar GS, Sudhakar P, Gupta N, Klopp N, Illig T, Söker T, Groth M, Platzer M, Gopinath PM, Graw J. Molecular analysis of cataract families in India: new mutations in the CRYBB2 and GJA3 genes and rare polymorphisms. Mol Vis. 2010 Sep 10;16:1837–1847.
- Wang KJ, Wang BB, Zhang F, Zhao Y, Ma X, Zhu SQ. Novel beta-crystallin gene mutations in Chinese families with nuclear cataracts. Arch Ophthalmol. 2011 Mar;129 (3):337–343.
- Lou D, Tong JP, Zhang LY, Chiang SW, Lam DS, Pang CP. A novel mutation in CRYBB2 responsible for inherited coronary cataract. Eye (Lond). 2009 May;23 (5):1213–1220.
- Pauli S, Söker T, Klopp N, Illig T, Engel W, Graw J. Mutation analysis in a German family identified a new cataract-causing allele in the CRYBB2 gene. Mol Vis. 2007 Jun 19;13:962–967.
- Hansen L, Mikkelsen A, Nürnberg P, Nürnberg G, Anjum I, Eiberg H, Rosenberg T. Comprehensive mutational screening in a cohort of Danish families with hereditary congenital cataract. Invest Ophthalmol Vis Sci. 2009 Jul;50 (7):3291–3303.
- Santhiya ST, Manisastry SM, Rawlley D, Malathi R, Anishetty S, Gopinath PM, Vijayalakshmi P, Namperumalsamy P, Adamski J, Graw J. Mutation analysis of congenital cataracts in Indian families: identification of SNPS and a new causative allele in CRYBB2 gene. Invest Ophthalmol Vis Sci. 2004 Oct;45 (10):3599–3607.
- Litt M, Carrero-Valenzuela R, LaMorticella DM, Schultz DW, Mitchell TN, Kramer P, Maumenee IH. Autosomal dominant cerulean cataract is associated with a chain termination mutation in the human beta-crystallin gene CRYBB2. Hum Mol Genet. 1997 May;6 (5):665–668.
- Mothobi ME, Guo S, Liu Y, Chen Q, Yussuf AS, Zhu X, Fang Z. Mutation analysis of congenital cataract in a Basotho family identified a new missense allele in CRYBB2. Mol Vis. 2009 Jul 30;15:1470–1475.
- Weisschuh N, Aisenbrey S, Wissinger B, Riess A. Identification of a novel CRYBB2 missense mutation causing congenital autosomal dominant cataract. Mol Vis. 2012;18:174–180.
- Liu BF, Liang JJ. Domain interaction sites of human lensbetaB2-crystallin. J Biol Chem 2006;281:2624–2630.
- Liedtke T, Schwamborn JC, Schröer U, Thanos S. Elongation of axons during regeneration involves retinal crystallin β b2 (crybb2). Mol Cell Proteomics 2007; 6:895–907.