Congenital cataract is the most common treatable cause of childhood blindness, which is characterized by opacification of all or part of the eye’s crystalline lens within the first year of life. The prevalence of congenital cataracts is 1 to 6 per 10,000 live births.Citation1 The cataract may be isolated, may be hereditary or secondary to an intrauterine event. Approximately one-quarter to one-third of congenital cataracts are inherited.Citation2,Citation3
Currently, four mutations in CRYGS associated with congenital cataract have been reported. Our present study (p. G57W) is the fifth report of a mutation in CRYGS linked with congenital cataract ().
TABLE 1. Summary of mutations in CRYGS responsible for congenital cataract.
A three-generation Chinese family with autosomal dominant congenital cataract from Anhui province was collected from Beijing Tongren Hospital. We also recruited 100 unrelated subjects without eye diseases except mild myopia from the Ophthalmology Clinic of Beijing Tongren Hospital as normal controls. The ethics committee of Capital Medical University approved the research. All participants from the family gave their informed consent. The study protocol followed the principles of the Declaration of Helsinki.
All 13 exon and intron-exon junctions of the candidate genes were amplified by polymerase chain reaction (PCR).Citation8 The sequencing results were analyzed using Chromas 2.33(http://www.technelysium.com.au/chromas.html).
Ten family members of a three-generation Chinese family with a history of cataracts participated in the study (five affected and five unaffected individuals). All patients in this family had bilateral cataracts. Most patients experienced decreased visual acuity at 3 years old, and then their visual acuity decreased gradually until surgery was required. A history of cataract extraction or ophthalmologic examination was used to determine the status affected, and ten family members participated in the study. The proband, who was a 7-year-old boy experienced a decrease in vision at 3 years old and had been diagnosed with bilateral cataracts at age 3. Slit-lamp examination revealed pulverulent cataract in the center. The boy’s best corrected visual acuity was 0.2/0.3. His 31-year-old mother also had pulverulent cataract in the center with peripheral cortical opacity. Her best corrected visual acuity was 0.3/0.4. Their clinical features were similar.
Through direct gene sequencing of the coding regions of the candidate genes, we identified a transversion of G > T at c. 169(p. G57W) in exon 2 of crystallin gamma S (CRYGS) in all affected individuals. However, we did not find this mutation in any unaffected family members or in the 100 unrelated controls. We found no further gene mutations in individuals from the studied family, except for a few nonpathogenic single nucleotide polymorphisms.
The c.169 G > T mutation resulted in a substitution of Glycine (Gly) with Tryptophan (Try) at the 57th amino acid position (p. G57W). Each single γ-crystallin domain consists of two Greek key motifs each containing four β-strands.Citation6 G57W is located in the NH2-terminal domain, domain 1, motif 2. CLC Main Workbench Software revealed that the Gly at the 57th amino acid position is highly conserved among many species. Furthermore, the second Greek key motif was found to be partially disrupted around the substitution site in the three-dimensional structural model of the mutant CRYGS by the SWISS-MODEL online tool (). In addition, the structure and function impact of the CRYGS G57W mutation was predicted by PolyPhen (http://genetics.bwh.harvard.edu/pph2/), and the result indicated that G57W could possibly be damaging, with a score of 1.000.
FIGURE 1. Protein models of the wild-type and mutant CRYGS. (A) A multiple-sequence alignment of the amino acid sequence in CRYGS from different species. The alignment data indicates that the Gly at the 57th amino acid position is highly conserved among many species (indicated by an arrow). (B) A structural model of the wild-type CRYGS is displayed. (C) A structural alteration of the mutant CRYGS is displayed. The second Greek key motif was found to be partially disrupted around the substitution site in the three-dimensional structural model of the mutant CRYGS.
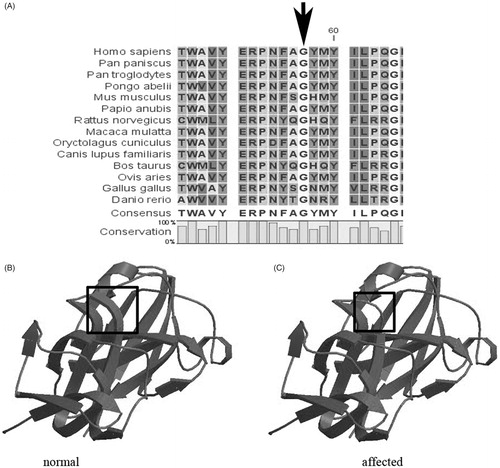
CRYGS is well-conserved in evolution and is expressed at high levels in human lens, particularly in adults in the cortical region.Citation9,Citation10 A G18V point mutation in CRYGS has previously been identified in affected members of a Chinese family leading to progressive cortical cataracts.Citation4 In our study, both the proband and his mother had pulverulent cataract in the center, but with age his mother also had cortical cataract in the periphery, which was not found in the proband. So we speculated that the mutation (G57W) in CRYGS may cause progressive cortical cataract.
In conclusion, the novel mutation (G57W) in CRYGS in this Chinese family is associated with autosomal dominant pulverulent cataract. The possible influence of the mutation on the structure as well as the function of CRYGS will require further investigation.
Acknowledgments
We thank the patients and their families for participation in this study.
Siquan Zhu ([email protected]) and Xu Ma ([email protected]) contributed equally to the research project and can be considered as equal co-corresponding authors.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This research was supported by grants from the Central Public-interest Scientific Institution Basal Research Fund [Grant No. 2010GJSSJKA07].
References
- Holmes JM, Leske DA, Burke JP, Hodge DO. Birth prevalence of visually significant infantile cataract in a defined U.S. population. Ophthalmic Epidemiol 2003;10:67–74
- Francis PJ, Moore AT. Genetics of childhood cataract. Curr Opin Ophthalmol 2004;15:10–15
- Vanita, Singh JR, Singh D. Genetic and segregation analysis of congenital cataract in the Indian population. Clin Genet 1999;56:389–393
- Sun H, Ma Z, Li Y, et al. Gamma-S crystallin gene (CRYGS) mutation causes dominant progressive cortical cataract in humans. J Med Genet 2005;42:706–710
- Vanita V, Singh JR, Singh D, et al. A mutation in GJA8 (p.P88Q) is associated with “balloon-like” cataract with Y-sutural opacities in a family of Indian origin. Mol Vis 2008;14:1171–1175
- Sun W, Xiao X, Li S, et al. Mutation analysis of 12 genes in Chinese families with congenital cataracts. Mol Vis 2011;17:2197–2206
- Vanita V, Singh JR, Singh D, et al. Novel mutation in the gamma-S crystallin gene causing autosomal dominant cataract. Mol Vis 2009;15:476–481
- Yang Z, Li Q, Ma Z, et al. A G–>T splice site mutation of CRYBA1/A3 associated with autosomal dominant suture cataracts in a Chinese family. Mol Vis 2011;17:2065–2071
- Wistow G, Bernstein SL, Wyatt MK, et al. Expressed sequence tag analysis of adult human lens for the NEIBank Project: over 2000 non-redundant transcripts, novel genes and splice variants. Mol Vis 2002;8:171–184
- Wistow G, Sardarian L, Gan W, Wyatt MK. The human gene for gammaS-crystallin: alternative transcripts and expressed sequences from the first intron. Mol Vis 2000;6:79–84
