Abstract
The present studies evaluate the antioxidant and vasorelaxant activities of the methanol stem bark extract of Turraeanthus africanus (Welw.) Pellegr. (Meliaceae). The antioxidant property investigated through the DPPH scavenging activity showed that the methanol extract from the stem bark of T. africanus was active with an inhibition rate of 72.47% at 500 μg/mL and IC50 of 29.2 μg/mL. This extract (0.1–700 μg/mL) induced a concentration-dependent relaxation of guinea-pig aortic rings precontracted with noradrenaline or KCl, with a maximum relaxation reaching 87.55 and 97.42%, respectively, at a concentration of 700 μg/mL. The extract showed no significant effect on the electrically induced contraction of the guinea-pig papillary muscle. The results of this study indicate that the extract from the stem bark has interesting antioxidant and vasorelaxant properties and represents a potential source of medicine for the treatment of cardiovascular diseases.
Introduction
Cellular damage or oxidative injury arising from free radicals or reactive oxygen species now appears to be the fundamental mechanism underlying a number of human neurodegenerative disorders, diabetes, inflammation, heart diseases, viral infections, autoimmune pathologies, and digestive system disorders. Free radicals are generated through normal metabolism of drugs, environmental chemicals, and other xenobiotics as well as endogenous chemicals, especially stress hormones (adrenaline and noradrenaline) Citation(Masuda et al., 2003). The antioxidants present in the human diet or in medicinal plants may play an important role in disease prevention (CitationSteinmetz & Potter, 1996; CitationAruoma, 1998). Many compounds extracted from plants have shown vasorelaxant activity (CitationYoshikawa et al., 1996; CitationDongmo et al., 2007) and antioxidant activity (CitationXiong et al., 1996; CitationThaipong, 2005). In Cameroon, the stem bark of Turraeanthus africanus (Welw.) Pellegr. (Meliaceae) is used in the treatment of intestinal worms, headaches, constipation, and inflammatory and cardiovascular diseases (CitationTongo & Ekwalla, 2003). This plant has a rather extensive range in Africa, from Sierra Leone westward to the Congo region and southward to Zaire and Angola. It is most common in the eastern region of the Ivory Coast and is scattered elsewhere. It is usually found in dense stands in rainforests, and along lakesides and streams (CitationKline, 1979; CitationKaiser, 1990). T. africanus is a medium-sized tree of the rainforest where it forms fairly dense but localized and discontinuous timber stands. Species of the Meliaceae family are well known for the production of terpenoid and limonoid compounds (CitationKimbu et al., 1984; CitationAyafor et al., 1994; CitationTane et al., 2004). Recently, studies carried out by CitationVardamides et al. (2006) on T. africanus led to the isolation of two new alkaloids, 10-O-demethyl-17-O-methyl isoarnottianamide and 11-demethoxyl-12-methoxyl oxynitidine, together with known compounds such as sesamin, decarine, and oxynitidine.
In continuation of our pharmacological studies on bioactive constituents of African medicinal plants (CitationSautour et al., 2004; CitationDongmo et al., 2005, Citation2007), and considering the claimed ethnopharmacological potency of T. africanus in the management of cardiovascular disorders (CitationTongo & Ekwalla, 2003), we are reporting here our findings on the antioxidant and vasorelaxant activities of the methanol stem bark extract of Turraeanthus africanus.
Materials and methods
Plant material
The stem bark of T. africanus was collected in August 2003 at Bonepoupa-Douala (Cameroon). The sample was identified by Dr. L. Zapfack, Botanic Department, University of Yaoundé I, where a voucher specimen is deposited for future reference.
Extraction
The air-dried powdered stem bark of T. africanus (8 kg) was macerated with methanol at room temperature for 48 h. The extract was concentrated under vacuum to produce a viscous brown oil (150 g). This extract was assigned for pharmacological tests.
In order to identify the main chemical groups, the MeOH extract was chromatographed by TLC (thin layer chromatography; silica gel plates 60 F254) using CHCl3–MeOH–H2O (65:35:10) as the mobile phase. TLC plates were observed under ultraviolet light (UV254nm) before and after spraying with Komarowsky reagent, prepared as described by CitationWagner and Bladt (1996), then heating. Another TLC (silica gel) procedure was performed on the methanol extract using toluene–EtOAc–diethylamine (70:20:10) as the mobile phase and spraying with modified Dragendorff reagent (CitationWagner & Bladt, 1996) for the detection of alkaloids.
Animals
Guinea-pigs (200–250 g) and Wistar rats (150–200 g) of either sex, obtained from the animal house of the Institute of Pharmacology and Toxicology of the Technical University of Munich, were used in this study. The animals were fed with pellet food and water ad libitum. In all animal experiments, the guidelines of the Animal Experimentation Ethics Committee, International Centre for Diarrheal Disease Research, Bangladesh (ICDDR, B) were followed.
Pharmacological investigations
Evaluation of radical scavenging activity
Qualitative determination by thin layer chromatography (TLC) The antioxidant activity was evaluated by measuring the reduction of the free radical 1,1-diphenyl-1-picryl-hydrazyl (DPPH). Extract (0.4 mg in 10 μL) was applied on a TLC plate (silica gel 60 F254, 0.2 mm thickness; Merck), developed in CHCl2–MeOH (9:1) or in AcOEt–HCOOH–CH3COOH–H2O (100:11:11:26), and sprayed with an ethanol solution of DPPH at a concentration sufficient to give a violet color to the plate (approximately 0.4 mg/mL). Sample containing radical scavengers was identified by yellow spots on the plate (CitationXiong et al., 1996).
Quantitative determination A stock solution ( 5 mg/mL) of the crude extract was prepared in ethanol. Serial dilutions were carried out to obtain concentrations of 1, 5, 10, 50, 100, and 500 μg/mL. Diluted solutions (1 mL each) were mixed with an ethanol solution of DPPH (0.004%, 1 mL) and allowed to stand at room temperature for 30 min. The UV absorbance of the resulting solutions was recorded at λ = 517 nm (CitationYan et al., 1998). The experiment was performed in triplicate and the average absorption was noted for each concentration.
Ascorbic acid was used as the positive control. The free radical scavenging activity was calculated as percentage inhibition of the DPPH radical by a sample or ascorbic acid according to the formula (CitationShilpi et al., 2006):
where AC(o) is the absorbance of the background control at t = 0 min and A(t) is the absorbance of the solution containing the antioxidant at a particular concentration at t = 30 min. The IC50 value is the concentration of the sample required to scavenge 50%.
Cardiovascular activities
Effect of the extract on isolated aorta rings. Guinea-pigs weighing 250–300 g were killed by cervical dislocation. The thoracic aorta was quickly removed, freed from connective tissue, and cut into 2– 4 mm ring segments. The rings were suspended on a fine gold chain in a 5 mL tissue bath and connected to an isometric force transducer (Hottinger Baldwin Messtechnik, Darmstadt, Germany) for isometric force measurement at a resting force of 9.8 mN. The bath contained modified Krebs–Henseleit solution of the following composition (mM): NaCl, 115; KCl, 4.7; CaCl2, 2; NaHCO3, 25; KH2PO4, 1.2; MgCl2, 1.2; glucose, 11.5, which was stirred and aerated with 95% O2 and 5% CO2. The temperature was maintained thermostatically at 35°C. After 60 min of equilibration, with changes of physiological solution every 15 min, aortic contraction was elicited by adding 30 mM KCl to check the functional integrity of the aortic rings. Afterward, the bath solution was changed and the resting force adjusted until it reached a constant value.
The first experiment examined the relaxant effect of the MeOH extract of T. africanus on noradrenaline (NA) (10 mM) or KCl (30 mM) induced contraction of aortic rings. When the contractile response became stable, the extract was added in a cumulative manner to the organ bath solution. In a second series of experiments, the effects of cumulative concentrations of NA (10−8–10−4 M) and KCl (3–120 mM) in the presence or absence of the MeOH extract (300 μg/mL) were recorded and the corresponding concentration–response curves were plotted. Measurements of the signal or force of contraction were computed using an EDV program based on ASYST Software (Keithley, Germering, Germany).
Characterization of vasorelaxant action. In order to investigate the mechanisms underlying the vasorelaxant action of the MeOH extract of T. africanus, rat aortic rings were preincubated with either nitro-l-arginine methyl ester (L-NAME; 100 μM) or indomethacin (10 μM) for 30 min before addition of the noradrenaline (NA) and the extract (CitationAjay et al., 2003; CitationRodriguez-Cruz et al., 2003). The rat aorta rings were first constricted with NA (1 μM) to test the endothelial integrity with a single addition of acetylcholine (Ach; 1 μM). Only endothelium-intact rings, those exhibiting more than 50% relaxation to ACh, were used for the experiments (CitationAjay et al., 2003). The aortic rings were precontracted with NA (1 μM), and the relaxant responses to the MeOH extract of T. africanus at different concentrations (0.1–700 μg/mL) were recorded by adding cumulative concentrations to the organ bath. Concentration–response curves were plotted as percent relaxation against logarithmic concentration of the MeOH extract of T. africanus solution.
Trials on papillary muscle Guinea-pigs were killed by cervical dislocation, and the heart quickly removed and immersed in Krebs–Henseleit solution ([Ca2+] = 3.2 mM). Thin papillary muscles were isolated from the right ventricles of guinea-pig hearts. For isometric force measurements, they were mounted vertically in a two-chambered vessel (volume 50 mL) (CitationReiter, 1981). The muscles were allowed to equilibrate for 1 h at 1 Hz contraction frequency in a solution of the following composition (in mM): NaCl, 115; NaHCO3, 25; KCl, 2.8; KH2PO4, 1.2; CaC12, 3.2; MgSO4, 1.2; glucose, 10.0. The solution was stirred and aerated by a mixture of 95% O2 and 5% CO2. The temperature was kept constant at 35°C. During this equilibration period the bath solution was changed 3–4 times. The resting force of the muscles was kept at 3.9 mN. Isometric contraction curves were monitored on a digital oscilloscope and recorded to a chart recorder by means of an inductive force transducer (Hottinger Baldwin Messtechnik). The extract (10–300 μg/mL) or nifedipine (10– 300 nM) was added to the organ bath in a cumulative manner.
Statistical analysis
All data are expressed as mean ± SEM for six experiments. The significance of differences was evaluated by means of one-way analysis of variance (ANOVA) followed by Dunnett’s comparison test in order to compare two groups (control versus experimental); p values lower than 0.05 were considered significant. EC50 values were calculated using a computer program: GraphPad Prism 3.00
Results
Phytochemical analysis
After spraying the TLC plate with Komarowsky reagent, many blue-violet zones were observed, indicating the presence of a number of terpenoids. The alkaloids were detected by modified Dragendorff reagent as orange-brown zones, which appeared immediately on spraying.
Radical scavenging activity
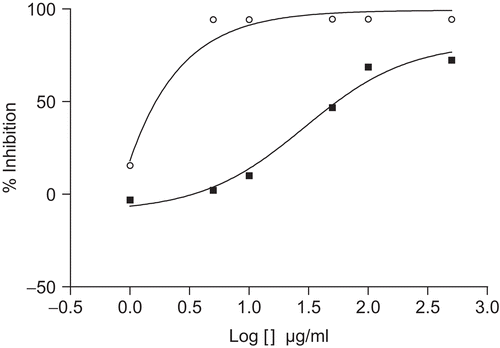
In the TLC-based qualitative antioxidant assay using DPPH spray, the methanol extract of T. africanus showed the presence of a number of yellow spots on a purple background on the TLC plates, indicating the free radical scavenging properties. The range of 1–500 μg/mL of this extract showed a concentration-dependent radical scavenging activity by inhibiting DPPH with an IC50 value of 29.2 μg/mL (). The reactivity of ascorbic acid, used as the positive control in this test, was substantially more powerful than the reactivity of the plant extract, showing an IC50 value of 4 × 10−3 μg/mL.
Vascular smooth muscle effect
The effects of the MeOH extract of T. africanus were studied on aortic rings precontracted with KCl (30 mM). The extract (1–700 μg/mL) induced a concentration-dependent relaxation of guinea-pig aortic rings (). The maximum relaxant effect reached was 87.5 ± 4.7% at a concentration of 700 μg/mL, with an EC50 of 173.6 μg/mL.
Figure 2. Concentration–response curves for relaxation induced by T. africanus extract on noradrenaline (NA) (♦) or KCl (•) precontrated aorta. The responses are expressed as % of maximum NA (10 μM) or KCl (30 mM) induced contraction. Values are mean ± SEM, n = 6.
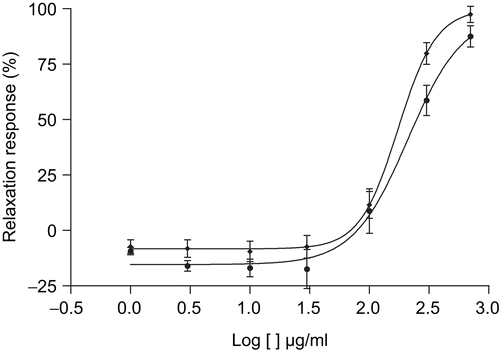
The extract, after cumulative addition into the organ bath (1–700 μg/mL), provoked concentration-dependent relaxation of the NA-induced contractile response (). The maximum relaxation force reached was 97.4 ± 3.6% of the reference contraction (100%) induced by NA (10 μM), at a bath concentration of 700 μg/mL of extract. The EC50 was 209.9 μg/mL.
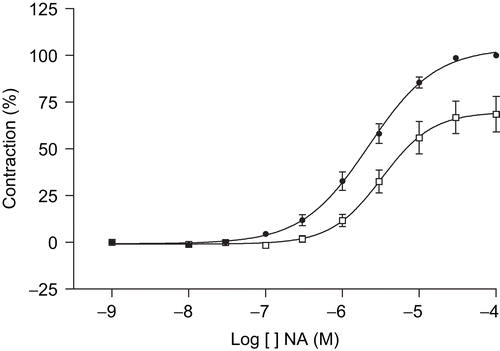
In order to compare the effect of the MeOH extract of T. africanus on the NA concentration–contraction response, the effect of the cumulative addition of concentrations of NA (10−8–10−4 M) on aortic rings was recorded in the absence (control) or presence of the extract (300 μg/mL). The contraction induced by a bath concentration of 10−4 M of NA in the control test was considered as the maximum reference contraction (100%). Pretreatment of the aortic rings with 300 μg/mL of extract of T. africanus provoked a shifting of the concentration–response (cumulative concentrations of NA, 10−8–10−4 M) curve about 1.5 times to the right, with a significant reduction of the maximum contraction to 68.5 ± 9.5% (). The EC50 values in the absence or presence of the extract were 2.20 × 10−6 M (95% confidence limits 1.7 × 10−6–2.6 × 10−6 M) and 3.26 × 10−6 M (95% confidence limits 2.0 × 10−6–5.2 × 10−6 M), respectively.
Figure 3. Contractile concentration–response curves of guinea-pig aorta rings to cumulative addition of noradrenaline (NA) in the presence (•) or absence (□) of MeOH extract of T. africanus (300 μg/mL). Contraction is expressed as percentage of the maximal contraction (100%) induced by NA (10–4 M). Mean ± SEM, n = 6.
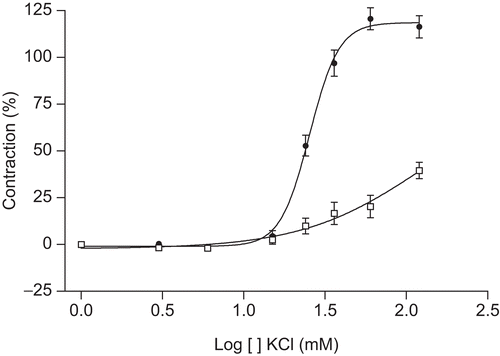
The contractile effect of the cumulative addition of concentrations of KCl (3–120 mM) on aortic rings was recorded in the absence (control) or presence of the extract (300 μg/mL). The contraction induced by a final organ bath concentration of 60 mM KCl in the control test was considered as the reference contraction (100%). Pretreatment of the aortic rings with 300 μg/mL of extract provoked a shifting of the concentration–response curve about four times to the right (). The EC50 values in the absence or presence of the extract were 123.1 and 35.41 mM, respectively.
Figure 4. Contraction concentration–response curves of guinea-pig aorta rings to cumulative addition of KCl in the presence (•) or absence (□) of MeOH extract of T. africanus (300 μg/mL). Contraction is expressed as percentage of the maximal contraction (100%) induced by KCl (60 mM). Mean ± SEM, n = 6.
Characterization of vasorelaxant action
To further determine the significance of the endothelium mechanisms in the vasorelaxant activity of the MeOH extract of T. africanus, the aortic rings were pretreated with a nitric oxide synthase (NOS) inhibitor, Nω-nitro-l-arginine methyl ester (l-NAME), and a cyclooxgenase inhibitor, indomethacin (10 μM). As shown in , L-NAME (100 μM) produced inhibition to the extract by giving an EC50 value of 101.9 μg/mL, compared to that of the control of 49.5 μg/mL (p < 0.05) as well as a slight changing of Emax (). This pretreatment with L-NAME provoked a shifting to the left of the concentration–response curve of about two times.
Figure 5. Vasorelaxant effect of MeOH extract of T. africanus-induced contractions of rat aorta rings with endothelium intact (♦) in the presence of indomethacin (10 μM, •) or l-NAME (100 μM, □). Mean ± SEM, n = 6.
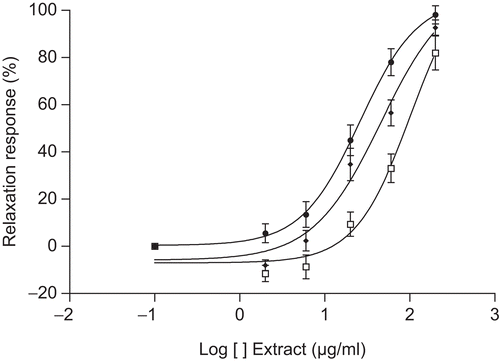
Table 1. Effect of l-NAME and indomethacin on maximal relaxant responses and EC50 of the MeOH extract of T. africanus against NA-induced contractions.
also shows the failure of an effect of indomethacin (10 μM) on the relaxant activity of the extract. The EC50 value (35.3 μg/mL) was similar to that of the control (49.5 μg/mL), and no change in the Emax value was observed (96.75 ± 3.86) compared to the control (92.6 ± 3.25).
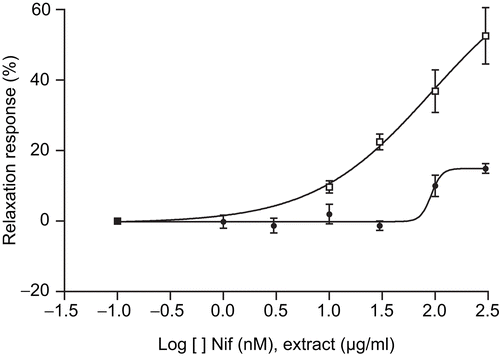
Effect of extract on guinea-pig papillary contraction
The MeOH extract of T. africanus was tested on the contraction of the papillary muscle. As shown in , the extract (0.1–100 μg/mL) was found to produce no significant modification on the contraction force of the guinea-pig papillary muscle, while this contraction was significantly and progressively inhibited by nifedipine (10–300 nM), a reference compound.
Discussion
In this work, the effects of the methanol extract of T. africanus were studied in several pharmacological experiments. This paper presents the cardiovascular and the antioxidant effect of the methanol extract of T. africanus.
The MeOH extract of the stem bark of T. africanus showed a concentration-dependent vasorelaxant effect. At a concentration of 700 μg/mL the relaxing effect reached 87.56 and 97.42% on KCl- as well as NA-induced contraction, respectively. It is known that KCl acts through activation of voltage-dependent calcium channels to induce the activation of voltage-dependent Ca2+ contraction (CitationKaraky & Weiss, 1984). Furthermore, stimulation of αl receptors by noradrenaline activates the G-protein related Ca2+ pathway through receptor-operated channels (CitationBülbring & Tomita, 1987). Thus, the vasorelaxant effects of T. africanus might well be related to the inhibition of voltage-dependent calcium channels, although mechanisms related to the signal transduction pathway coupled to αl adrenoceptors could also be considered.
Aortic rings pretreated with the MeOH extract of T. africanus provoked a shift of the KCl-induced concentration–response curve to the right, with an important modification of the maximum effect. The similar shift of the NA-induced concentration–response curve was accompanied by a significant reduction of the maximum contraction. This effect denotes a non-competitive inhibition by the extract.
Vascular tone is regulated by a number of receptor- and ion channel-mediated processes, and the contribution of an intact vascular endothelium is considered important. Modulation of vascular tone by the endothelium is regulated by the synthesis and release of vasorelaxing factors such as nitric oxide and prostacyclin, as well as by vasoconstricting factors such as endothelin and angiotensin II (CitationRubanyi, 1993). The inhibiting effect of l-NAME on the vasorelaxant property of the plant extract further supported that nitric oxide, an endothelium-derived relaxant factor (EDRF), was involved in the T. africanus-mediated vasorelaxant effect.
In addition to nitric oxide, prostaglandins (PGs) such as prostacyclin constitute another group of EDRFs. However, indomethacin [a cyclooxygenase (COX) inhibitor] had no significant effect on the extract-induced vasodilator response, suggesting that production of prostanoids by the endothelial cells may not be relevant to the action of the extract.
Several compounds have been isolated from T. africanus, amongst which is sesamin (lignan). This last compound has been shown to be useful as a prophylactic treatment in the development of hypertension, endothelial dysfunction, and prevention of atherosclerosis (CitationNakano et al., 2004). Our preliminary test showed that this compound induced a relaxation on aorta precontracted with noradrenaline (results not shown). This finding has also been reported by CitationNakano et al. (2006), who suggested that the enhancement of endothelium-dependent vasorelaxation induced by sesamin metabolites is one of the important mechanisms of the in vivo antihypertensive effect of sesamin. Moreover, it has been demonstrated that T. africanus contains some terpenoid compounds (CitationKimbu et al., 1984; CitationAyafor et al., 1994). It has also been previously demonstrated that some terpenoids possess vasodilator properties, which might be also responsible for the observed effects of the plant extract (CitationDuarte et al., 1992; CitationYoshikawa et al., 1996; CitationAndre et al., 1999; CitationGuerrero et al., 2004).
We also investigated the effect of the extract on the electrically induced contraction of the guinea-pig papillary muscle, which depends on the calcium influx. The results were compared with those obtained with nifedipine, a dihydropyridine which is an antagonist of L-type calcium channels. Only the reference compound nifedipine exerted negative inotropic effects in heart muscle, whereas the extract showed no significant effect. The decrease in contractile force induced by nifedipine is due to inhibition of the calcium channels. It is suggested that the extract may be acting preferentially on vascular smooth muscle rather than on cardiac muscle.
In this study, the antioxidant capacity of the MeOH extract of T. africanus was tested using the DPPH assay. Ethanol solutions of the stable 1,1-diphenyl-1-picryl-hydrazyl (DPPH) radical strongly absorbed at 517 nm, showing a deep purple color. These solutions in the presence of any test substance with free radical scavenging (antioxidant) activity can be “decolorized,” resulting in bleaching on the TLC plate and a reduction in absorbance measured spectrophotometrically. T. africanus extract showed DPPH free radical scavenging activity in a concentration-dependent manner with an IC50 value of 29.2 μg/mL. Many pharmacological studies have demonstrated the ability of medicinal plants to exhibit antioxidant activity. Among these plants we can cite, for example, Hypericum triquetrifolium and Hypericum scabroides. Both plant extracts tested were found to be highly active in the DPPH radical scavenging assay with an IC50 of 39.0 and 33.8 μg/mL, respectively (CitationKızıl et al., 2008). The antioxidants present in the human diet or in plant extracts may play an important role in disease prevention (CitationSteinmetz & Potter, 1996; CitationAruoma, 1998). These antioxidant properties are generally associated with phenolic compounds such as protocatechuic acid, ferulic acid, quercetin, and guavin B (CitationThaipong et al., 2005), but can also be attributed to the presence of other compounds such as triterpenoids (CitationGao et al., 2006, CitationBackhouse et al, 2008).
A recent study demonstrated that sesamin and its metabolites had in vitro radical scavenging activities (CitationNakano et al., 2006). This compound (sesamin, a lignan) and terpenoids present in T. africanus extract may be responsible in part for its antioxidative activity. Thus, the antioxidative effect of sesamin metabolites modulates the vascular tone and contributes to the in vivo antihypertensive effect of sesamin (CitationNakano et al., 2006). The high reactivity and the potent free radical scavenging activity of V. cienkowskii observed may explain, at least in part, its cardiovascular potency.
The results of this study indicate that the extract from the stem bark has interesting antioxidant and vasorelaxant properties and represents a potential source of medicine for the treatment of cardiovascular diseases. Further investigations will be necessary to confirm the action mechanisms and to isolate the compounds responsible for the observed pharmacological effects.
Acknowledgements
The authors wish to thank the University of Douala for financial assistance and the DAAD (German Academic Exchange Service) for the equipment grant allocated to one of the authors (A.B.D).
Declaration of interest: The authors report no conflicts of interest.
References
- Ajay M, Gilani AH, Mustafa MR (2003): Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci 74: 603–612.
- Andre E, Malheiros A, Cechinel-Filho V, Yunes RA, Calixto JB (1999): Mechanisms underlying the relaxation caused by the sesquiterpene polygodial in vessels from rabbit and guinea-pig. Eur J Pharmacol 10: 47–53.
- Aruoma OI (1998): Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc 75: 199–212.
- Ayafor JF, Kimbu SF, Ngadjui BT, Akam TM, Dongo E, Sondengam BL, Connolly JD, Rycroft DS (1994): Limonoids from Carapa grandiflora (Meliaceae). Tetrahedron 50: 9343–9354.
- Backhouse N, Rosales L, Apablaza C, Göity L, Erazo S, Negrete R., Theodoluz C, Rodriguez J, Delporte C (2008): Analgesic, anti-inflammatory and antioxidant properties of Buddleja globosa, Buddlejaceae. J Ethnopharmacol 116: 263–269.
- Bülbring E, Tomita T (1987): Catecholamine action on smooth muscle. Pharmacol Rev 39: 49–96.
- Dongmo AB, Nguelefack T, Lacaille-Dubois MA (2005): Antinociceptive and anti-inflammatory activities of Acacia pennata Willd (Mimosaceae). J Ethnopharmacol 98: 201–206.
- Dongmo AB, Azebaze AGB, Meyer M, Sontia B, Ouahouo B, Nkengfack AE, Vierling W (2007): Vasodilator effect of the extracts and some coumarins from the stem bark of Mammea africana (Guttiferae). J Ethnopharmacol 111: 329–334.
- Duarte DF, Sant’Ana AE, Calixto JB (1992): Analysis of the vasorelaxant action of jatrophone in the isolated aorta of the rat: Influence of potassium channel blockers. Eur J Pharmacol 29: 75–81.
- Gao J, Chen J, Tang X, Pan L, Xu L, Zhao L, Xu Q (2006): Mechanism underlying mitochondrial protection of asiatic acid against hepatotoxicity in mice. J Pharm Pharmacol 58: 227–233.
- Guerrero MF, Puebla P, Carron R, Martin ML, San Roman L (2004): Vasorelaxant effect of new neo-clerodane diterpenoids isolated from Croton schiedeanus. J Ethnopharmacol 94: 185–189.
- Karaky H, Weiss GB (1984): Calcium channels in smooth muscle. Gastroenterology 87: 960–970.
- Kaiser J (1990): Wood of the month – avodire: The light African wood with hidden strength. In: Kaiser J, ed., Wood of the Month, Supplement to Wood and Wood Products. New York, p. 22.
- Kimbu SF, Ayafor JF, Sondengam BL, Tsamo E, Connolly JD, Rycroft DS (1984): Carapolides D, E, F, novel tetranortriterpenoids from the seeds of Carapa grandiflora (Meliaceae). Tetrahedron Lett 25: 1617–1620.
- Kızıl G, Kızıl M, Yavuz M, Emen S, Hakimoglu F (2008): Antioxidant activities of ethanol extracts of Hypericum triquetrifolium and Hypericum scabroides. Pharm Biol 46: 231–242.
- Kline M (1979): Turraeanthus africanus – Avodire. In: Flynn JH, ed., A Guide to Useful Woods of the World. Portland, ME, King Philip Publishing Co., p. 354.
- Masuda T, Inaba Y, Maekawa T, Takeda Y, Yamaguchi H, Nakamoto K, Kuninaga H, Nishizato S, Nonaka A (2003): Simple detection method of powerful antiradical compounds in the raw extract of plants and its application for the identification of antiradical plants constituents. J Agr Food Chem 51: 1831–1838.
- Nakano D, Takaoka M, Kiso Y, Matsumura Y (2004): Antihypertensive effect of sesamin. Vasc Dis Prev 1: 233–241.
- Nakano D, Kwak CJ, Fujii K, Ikemura K, Satake A, Ohkita M, Takaoka M, Ono Y, Nakai M, Tomimori N, Kiso Y, Matsumura Y (2006): Sesamin metabolites induce an endothelial nitric oxide-dependent vasorelaxation through their antioxidative property-independent mechanisms: Possible involvement of the metabolites in the antihypertensive effect of sesamin. J Pharmacol Exp Ther 318: 328–335.
- Reiter M (1981): The use of the isolated papillary muscle for the evaluation of positive inotropic effects of cardioactive steroids. In: Greef K, ed., Handbook of Experimental Pharmacology. Berlin, Springer-Verlag, p. 153.
- Rodriguez-Cruz ME, Pérez-Ordaz L, Serrato-Barajas BE, Juárez-Oropeza MA, Mascher D, Paredes-Carbajal MC (2003): Endothelium-dependent effects of the ethanolic extract of the mistletoe Psittacanthus calyculatus on the vasomotor responses of rat aortic rings. J Ethnopharmacol 83: 213–218.
- Rubanyi GM (1993): The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol 22: S1–S14.
- Sautour M, Mitaine-Offer AC, Miyamoto T, Dongmo A, Lacaille-Dubois MA (2004): Antifungal steroid saponins from Dioscorea cayenensis. Planta Med 70: 90–92.
- Shilpi JA, Taufiq-Ur-Rahman M, Uddin SJ, Alam MS, Sadhu SK, Seidel V (2006): Preliminary pharmacological screening of Bixa orellana L. leaves. J Ethnopharmacol 108: 264–271.
- Steinmetz KA, Potter JD (1996): Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc 96: 1027–1039.
- Tane P, Akam MT, Tsopmo A, Ndi CP, Sterner O (2004): Two labdane diterpenoids and a seco-tetranortriterpenoid from Turraeanthus africanus. Phytochemistry 65: 3083–3087.
- Thaipong K, Boonprakob U, Cisneros-Zevallos L, Byrne DH (2005): Hydrophilic and lipophilic antioxidant activities of guava fruits. Southeast Asian J Trop Med Public Health 36: 254–257.
- Tongo J, Ekwalla E (2003): Nos plantes qui soignent Centre Spirituel de Rencontre. Basel, Switzerland, Roche, p. 38.
- Vardamides JC, Dongmo AB, Ndom JC, Azebaze AGB, Zounda MRS, Nkengfack AE, Ngando TM, Fomum ZT, Meyer M (2006): Alkaloids from the stem bark of Turraeanthus africanus (Welw. Ex. C.D.C.) Pellegr. Chem Pharm Bull 54: 1034–1036.
- Wagner H, Bladt S (1996): Plant Drug Analysis, 2nd ed. Berlin, Springer-Verlag, pp. 360–362.
- Xiong Q, Kadota S, Tani T, Namba T (1996): Antioxidative effects of phenylethanoids from Cistanche deserticola. Biol Pharm Bull 19: 1580–1585.
- Yan X, Nagata T, Fa X (1998): Antioxidative activities in some common seaweeds. Plant Foods Hum Nutr 52: 253–262.
- Yoshikawa M, Shimada H, Matsuda H, Yamahara J, Murakami N (1996): Bioactive constituents of Chinese natural medicines. I. New sesquiterpene ketones with vasorelaxant effect from Chinese moxa, the processed leaves of Artemisia argyi Levl. et Vant.: Moxartenone and moxartenolide. Chem Pharm Bull 44: 1656–1662.