Abstract
Houttuynia cordata Thunb. is a member of Saururaceae, a family mainly distributed in Eastern Asia. It has long been used in China both as an edible vegetable and in traditional medicine. Recent studies indicate that H. cordata contains abundant nutrients and active components including volatile oils, flavonoids, and water soluble polysaccharides. In addition, H. cordata exhibits a wide range of pharmaceutical activities including antibacterial, antiviral, anti-inflammatory, immunologic, anticancer, antioxidative, and antimutagenic effects. At present, injectable H. cordata has been used clinically for treating infectious disease, inflammation, and anaphylaxis. This paper provides a comprehensive review of the nutrients and pharmacologically relevant compounds of H. cordata that have been characterized to date, and of the studies supporting its medicinal use. Particular attention has been given to the pharmacological action and the state of utilization of H. cordata. Finally, future trends on H. cordata such as pharmacological components and mechanism, and the development of potential products have briefly been inferred.
Introduction
The Saururaceae family contains four genera and six species. Houttuynia cordata Thunb. (H. cordata), a perennial herb, is the sole member of the genus Houttuynia (CitationLiang, 1995). H. cordata reaches an average length of 15-50 cm, and has a thin stalk and heart-shaped leaf. It is usually harvested in summer or autumn when its stalk and leaves mature. It is distributed mainly in Eastern Asia. Within China, it occurs mainly in the central, southeast and southwest regions (CitationYang, 2003).
H. cordata contains a number of mineral nutrients (essential to the human body) as well as other active components – specifically, high levels of volatile oils and flavonoids (CitationZheng & Li, 2002). The Chinese Ministry of Health included it the list of medicine and food in 1998 (CitationLi, 2001). Since ancient times, H. cordata has been used in China as an edible vegetable and effective traditional Chinese medicine (TCM). It has a wide range of benefits including an antileukemic effect (CitationKwon et al., 2003), antimutagenic effect (CitationChen et al., 2003), anti-inflammatory action (CitationChiang et al., 2003), and antianaphylaxis effect (CitationLi et al., 2005), as well as the ability to promote immunologic function. CitationHayashi et al. (1995) reported on its antileukemic activity and virucidal effects on the human immunodeficiency virus (HIV).
In China, the Science and Technology Group of the National Headquarters for SARS Prevention and Control was set up on April 25, 2003. On May 23, 2003, Chinese scientists affirmed eight types of traditional Chinese medicine that had been shown to play a role in reducing the side-effects of western medicine and improving the immune systems of patients infected with severe acute respiratory syndrome (SARS). The eight, including injections of H. cordata, were found effective in curbing lung inflammation. In addition, H. cordata reduced fever, chills, headaches, muscular pain, malaise, diarrhea, and dry tight coughs (CitationLu et al., 2006b).
More recently, H. cordata has become a candidate for treating SARS in China since preliminary experimental results indicated that H. cordata extracts can kill the SARS virus (CitationXu et al., 2005). Currently, H. cordata is used in clinical therapy as TCM to treat infectious disease, inflammation, and anaphylaxis. In order to fully utilize this widely available herb in areas such as the food and cosmetic industries, this review summarizes the progress of studies on the nutritional ingredients, active components, pharmacological actions, and the utilization of H. cordata.
Nutritional ingredients of H. cordata
Recently, research on the analysis of the nutrients in H. cordata, has reported amino acids, vitamins and trace elements such as potassium, zinc, iron, copper and manganese ( and ). The reported quantity of these elements varies depending on the harvesting season, cultivars, growing conditions and test facilities.
Table 1. Chemical components and contents of H. cordata.
Table 2. The contents of amino acids of H. cordata (CitationWu et al., 2000).
At least sixteen amino acids were found in H. cordata, of which six are essential amino acids. Zinc, iron, copper, and manganese as essential trace elements have an important physiologic function. So the herb is rich in nutritional ingredients which are conducive to health.
Active components in H. cordata
Medicinal plants typically contain several different bioactive compounds that may act individually, additively or in synergy to improve health (CitationGurib-Fakim, 2006). In recent decades, active components isolated from botanical sources have attracted great attention in the biomedical area because of their high effectiveness and low toxicity (CitationRen et al., 2004). Those found in H. cordata include volatile oils (decanoyl acetaldehyde, myrcene, lauric aldehyde, α-pinene, d-limonene, methyl nonyl ketone), organic acids (palmitic acid, linoleic acid, aspartic acid), flavonoids (quercetin, isoquercitrin, afzelin, hyperin, reyoutrin, rutin), cordarine, kalium sulfuricum, water-soluble polysaccharides (CitationLi et al., 2004; CitationChen et al., 2004; CitationCao & Wang, 2005).
Volatile oils
Volatile oils represent a small fraction of the composition of medicinal plants, but have a wide distribution in the vegetable kingdom. They exhibit a wide range of pharmaceutical activities such as anaphylaxis (CitationTanaka et al., 1996), enzyme inhibiting (CitationMiyazawa et al., 1997), antimutagenic (CitationMiyazawa et al., 1996), antimicrobial (CitationBarmba et al., 1993), antiviral (CitationEI-TE et al., 1994), insecticidal (CitationSharma et al., 1992), and a depressant activity on the central nervous system (CitationAnsari et al., 1993). CitationChen et al. (2005) reported that the volatile oils content of H. cordata originating from different collections, from wild and cultivated plants as well as from different regions (root, stalk, and leaf), ranged from 0.038 to 0.16 (mL/g). A main component in volatile oils, decanoyl acetaldehyde, has known pharmacological effects, but it is unstable and easily oxidized () (CitationZeng et al., 2003) during distillation and storage. Thus, it is crucial that the natural proportion of the components is maintained during extraction of the essence (). CitationLiang et al. (2005) compared three procedures (HS-SPME, FE and SD for Gas Chromatography and Mass Spectrometry (GC-MS) ) for extraction. He concluded that HS-SPME was the most selective and was particularly efficient in the isolation of volatile oils, obtaining a greater number of compounds than that of FE or SD. A total of 60 compounds were detected in SPME extracts while in FE and SD extracts, the detected compounds were 41 and 51, respectively. The total amount of compounds isolated by SPME was much larger than that isolated by FE or SD.
Figure 1. The decanoyl acetaldehyde can be converted into 2-undecanone via both oxidation and decarboxylation.
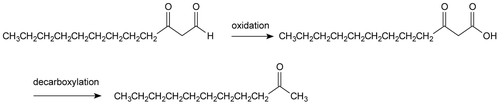
Table 3. Summary of main extraction techniques of volatile compounds from H. cordata.
Flavonoids
Flavonoids are natural products widely distributed in the vegetable kingdom and currently consumed in large amounts (± 1 g) in the daily diet (CitationCarlo et al., 1999). In citrus fruit they may represent up to 1% of the fresh fruit. Flavonoids are capable of modulating the activity of enzymes and affecting the behavior of many cell systems, suggesting that they may possess significant anti-hepatotoxic, antiallergic, anti-inflammatory, anti-osteoporotic and even anti-tumor activity.
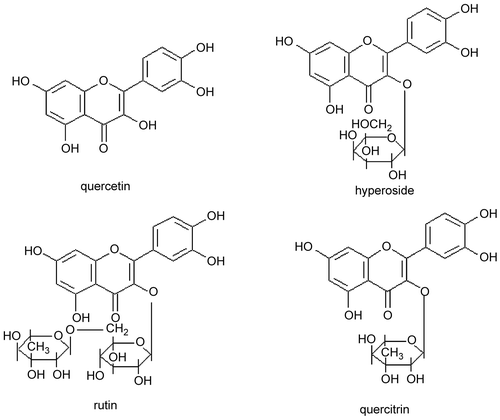
CitationGuo and Xu (2007) found that the yield of flavonoids in H. cordata reached 0.586%. Flavonoids have been identified as rutin, hypersoide, quercetin and quercitrin using analysis of their physical and chemical properties, Thin Layer Chromatography (TLC) and Ultraviolet-visible detection (UV-VIS). In a recent study, the molecular structures of the rutin, hyperoside, quercitrin and quercetin from flavonoids of H. cordata were estimated (). The content of the rutin, hyperoside, quercitrin and quercetin was 7.6%, 7.65%, 8.1% and 16.7% respectively. The leaves contained a higher content of the four flavonoids than the roots (CitationXu et al., 2006). CitationMeng et al. (2006) employed various columns including Diaion HP-20, Sephadex LH-20, ODS and silica gel for the isolation and purification of compounds from fresh H. cordata. The structures of the compounds were identified by physiochemical properties and spectral analysis. Five compounds were isolated and identified as (1) quercetin-3-O-β-d-galactoside-7-O-β-d-glucoside, (2) kaempferol-3-O-α-l-rhamnopyranosyl- (1 →6)-β-d-glucopyranoside, (3) quercitrin, (4) hyperin, (5) quercetin-3-O-α-l-rhamnopyranosyl-7-O-β-d-glucopyranoside. This was the first time that compounds (1), (2) and (5) were separated from H. cordata. In the literature, pressurized liquid extraction (PLE) (CitationZhang et al., 2007), hot soaking, macroporous exchange resin (CitationYou, 2006), and ultrasound-assisted extraction (CitationLi et al., 2006) have all been adopted to extract flavonoids from H. cordata.
Figure 2. The molecular structures of quercetin, hyperoside, rutin, and quercitrin (modified from CitationXu et al., 2006).
Water soluble polysaccharides
Polysaccharides from plant, epiphyte and animal extracts are an interesting source of additives, particularly for the food and drug industries (CitationLi et al., 2007). Botanical polysaccharides exhibit a number of beneficial therapeutic properties, and it is thought that the mechanisms involved in these effects are due to the modulation of innate immunity and, more specifically, enhanced an activated macrophage function. This leads to immunomodulation, anti-tumor activity, wound-healing and other therapeutic effects (CitationSchepetkin & Quinn, 2006). CitationZhang et al. (2000) identified the components of water-soluble polysaccharides extracted from H. cordata by polyacrylamide gel electrophoresis and thin layer chromatography. They were assessed to be a heteroglycan composed of glucose, fructose, arabinose, galactose, xylose, rhamnose and an unknown pentaglucose. CitationCheng & Li (2006) found that the polysaccharides of H. cordata could quench 50% of hydroxy and superoxide radicals at a concentration of 6 and 6.25 mg/mL respectively. Using conventional extraction technology, the yield of flavonoids and water soluble polysaccharides was relative low (CitationSheng et al., 1999; CitationZhang et al., 2000). However, CitationChen and Cheng (2005) introduced microwave-assisted complex extraction technology by which the extraction yields of all effective constituents and utilization rate of raw materials increased significantly.
Pharmacological action of H. cordata
Antibacterial action
A main component in H. cordata, decanoyl acetaldehyde, is known to have pharmacological effects. The two-fold broth dilution and agar dilution method was used to study all the essential oils of H. cordata for their antibacterial properties against Staphylococcus aureus and Sarcina ureae. The method determined the minimum inhibitory concentration (MIC) of essential oil from different species and parts of species. Results showed that all essential oils possessed antibacterial effect, with MIC values in the range of 0.0625 × 10(−3) to 4 × 10(−3) mL/mL (CitationLu et al., 2006a). CitationZhou et al. (2006) studied the bacteriostatic action of H. cordata by designing a L9 (34) extraction method, and using, petroleum ether as the solvent, and Staphylococcus aureus, Bacillus dysenteriae, Escherichia coli, yeast, fungus and Penicillium as the tested strains. While all parts of the herb exhibit bacteriostatic action, the extract from the root had stronger inhibition on Staphylococcus aureus, Escherichia coli, and yeast with MIC of 0.04, 0.06, 0.06 g/mL, respectively. The leaf extract had stronger inhibition on Bacillus dysenteriae, the MIC was 0.08 g/mL, all extracts had no obvious bacteriostatic action on Penicillium. At present, antiseptic drugs extracted from H. cordata have been widely used in clinical therapy.
Antiviral activity
H. cordata has an inhibitory action on several viruses. The steam distillate has direct inhibitory activity against herpes simplex virus type 1 (HSV-1), influenza virus, and human immunodeficiency virus type 1 (HIV-1) without showing cytotoxicity, but not against poliovirus and coxsackie-virus. The loss of viral infectivity was related to the duration of drug treatment. Three major components of the distillate, methyl n-nonyl ketone, lauryl aldehyde, and capryl aldehyde, also inactivated HSV-1, influenza virus, and HIV-1. The in vitro findings demonstrated that the essential oils provide virucidal activity against enveloped viruses by interfering with the function of the virus envelope (CitationHayashi et al., 1995). Based on the cytopathogenic effect, the extracts from H. cordata inhibited the replication of influenza virus A, B, and mumps (IFVA, IFVB, MPV). The lowest antiviral concentration needed for each was 4.5, 2.25 and 14 mg/mL, respectively (CitationLi et al., 1999). CitationChiang et al. (2003) evaluated the antiviral activity of H. cordata using a cytotoxicity test with an XTT-based colorimetric assay. BCC-1/KMC cells were infected with herpes simplex virus (HSV) and then were cultured with a hot water extract of H. cordata (HWHC). HWHC significantly inhibited the replication of HSV at a concentration of 250 μg/mL (10.2% for HSV-1, p < 0.05; 32.9% for HSV-2, p < 0.005). The ED50 of HSV type 1 (HSV-1) and HSV type 2 (HSV-2) for HWHCq were 822.4 and 362.5 μg/mL, respectively. Both drugs had selective indexes above 1.04. H. cordata was more effective against HSV-2 than against HSV-1, and had a low ED50 against HSV-2. This suggests that H. cordata might be useful against HSV-2.
Anti-inflammatory action
H. cordata injections (HCI) have been used as an anti-inflammatory in China. In order to validate this property, the inflammation induced by carrageenan in rat pleurisy and by xylene in mouse ear edema was adopted. Injection of carrageenan into the pleural cavity elicited an acute inflammatory response characterized by protein-rich fluid accumulation and leukocyte infiltration in the pleural cavity. The peak inflammatory response was obtained at 24 h when the fluid volume, protein concentration, C-reactive protein, and cell infiltration were the highest. The results showed that these parameters were attenuated by HCI at any dose and touched bottom at a dose of 0.54 mL/100 g. This drug was also effective in inhibiting xylene-induced ear edema, and the percentage of inhibition was 50% at a dose of 80 μL/20 g. These results confirm that HCI has anti-inflammatory activity (CitationLu et al., 2006b).
Synthetic houttuynin also showed a marked suppressive effect on the mouse ear edema induced by croton oil and the rat hind paw edema induced by carrageenan. It was also found to inhibit vascular permeability induced by acetic acid in mice. In addition, a significant analgesic effect was observed on inflammatory pain caused by acetic acid, formaldehyde and heat stimulus (CitationLi et al., 1998).
Immunologic function
Feeding decanoyl acetaldehyde to induced splenectomy immunodeficiency mice strengthened the phagocytosing function of the peritoneal macrophage system, delayed the typical hypersensitivity (DTH) reaction, and significantly enhanced the level of serum hemolysin and the ANAE (+) cell percentage of the peripheral blood lymphocytes. These results suggest that decanoyl acetaldehyde can strengthen non-specific and specific immune functions in splenectomized mice (CitationShao et al., 1999).
Antioxidative and antimutagenic effect
CitationChen et al. (2003) evaluated the antioxidative effect of H. cordata by subjecting rodents to oxidized frying oil-induced oxidative stress to examine the antimutagenic effects of H. cordata using the Ames test. Forty-eight Sprague-Dawley rats were fed a diet of 0%, 2%, or 5% of H. cordata and 15% fresh oil or oxidized frying oil (OFO) for 28 days. Levels of polyphenol in the feces, plasma, and liver were determined. The LDL lag time, plasma total antioxidant status (TAS), and levels of thiobarbituric acid-reactive substances (TBARS) were used as antioxidative indices, and the protein carbonyl group was used as an oxidative index. Results showed that the polyphenol content decreased in the plasma and increased in the feces when administering OFO, and the apparent absorption of polyphenol also decreased while the polyphenol content in plasma increased. There was a higher polyphenol concentration in the water extracts of H. cordata than in its methanol extracts. The OFO-fed groups had higher plasma TBARS and hepatic protein carbonyl group concentrations and shorter LDL lag times than those of the control group. The total TAS was elevated and the LDL lag time was prolonged when fed with H. Cordata. In addition, both water and methanol extracts of H. cordata had an antimutagenic effect on benzo(a)pyrene, aflatoxin B1, and OFO, and showed a dose-dependent response using the Ames test. In conclusion, the polyphenol in H. cordata was easily absorbed and metabolized by rodents. H. cordata showed both antioxidative and antimutagenic properties under OFO feeding-induced oxidative stress.
Anticancer effect
CitationKwon et al. (2003) investigated the cellular effects of H. cordata extract (HCE) and the signal pathways of HCE-induced apoptosis on the HL-60 human promyelocytic leukemia cell line. HCE treatment caused apoptosis of cells as evidenced by discontinuous fragmentation of DNA, the loss of mitochondrial membrane potential, release of mitochondrial cytochrome C into the cytosol, activation of procaspase-9 and caspase-3, and proteolytic cleavage of poly (ADP-ribose) polymerase. Pretreatment of Ac-DEVD-CHO, caspase-3 specific inhibitor, or cyclosporin A, a mitochondrial permeability transition inhibitor, completely abolished HCE-induced DNA fragmentation. Together, these results suggest that HCE possibly causes mitochondrial damage leading to cytochrome c release into cytosol and activation of caspases resulting in PARP cleavage and execution of apoptotic cell death in HL-60 cells. To evaluate the anti-leukemic activity of H. cordata, cytotoxicity tests with an XTT-based colorimetric assay were used. Five leukemic cell lines, namely L1210, U937, K562, Raji and P3HR1, were cultured with hot water extracts of H. cordata. The five lines were inhibited with IC50 between 478 and 662 μg/mL (CitationChang et al., 2001).
Application of H. cordata
Fresh herb of H. cordata
China has long kept the most extensive records on the use of H. cordata as a wild vegetable. Both the root and herb are edible and highly nutritious. As a supplementary vegetable, it has been increasingly consumed by more and more people. Usually, the herb is eaten raw or to flavor foods such as stewed meat, mixed flour, or cooked gruel to facilitate its use. After studying its practicality as a supplement, CitationZhou (2006a) studied H. cordata as a convenience food, while CitationZhou (2006b) introduced the processing technology of soft-packing flavored H. cordata, which will contribute to its marketing.
The processing of H. cordata
In recent years H. cordata has been used as a healthy additive in different products.
Utilization of H. cordata extracts
H. cordata has abundant nutritional ingredients, including volatile oils, flavonoid and water soluble polysaccharides, etc. which are beneficial if eaten regularly. At present, the extract is used in flavoring in various beverage products, including soft drinks, fermented drinks (CitationWu & Tang, 2001) and teas (CitationGong, 2003). CitationLiu and Xue (2006) proposed a composite health beverage made from the extracts of H. cordata and kudzuvine root. The best recipe was 40% each of H. cordata and kudzuvine root compound juice with 0.6% honey, 0.9% citric acid and 0.04% CMC-Na. Using H. cordata, girald acanthopanax bark and Glycyrrhiza uralensis as the main ingredients, CitationZhang et al. (2005) prepared a recipe for H. cordata tea, which had therapeutic effects such as heat-clearing and detoxifying, increasing lung moisture to arrest cough, eliminating rheumatism and strengthening bones and muscles.
Utilization of H. cordata herb
Using H. cordata as the main ingredient, CitationZhou (2007) studied processing it as a fried food. The fresh herbs were blanched for 1 min, dried for 120 min at 70°C, then fried for 2 min at 180°C, followed by removing excess oil, seasoning and vacuum packing. The final product retained the characteristic crispness and flavor of H. cordata. Furthermore, it was found that the problem of browning and wilting was solved by frying. Using fresh beef and H. cordata as the main ingredients, CitationZhan et al. (2006) studied beef jerky with H. cordata, which not only strengthened the flavor of the beef jerky, but added health benefits.
Utilization of H. cordata volatile oils
A main component in H. cordata, decanoyl acetaldehyde, is known to be antibacterial. Therefore, the extract can be used as a natural food preservative. Its use partially avoids sterilization which is beneficial to retain the nutrition and flavor of the food. CitationXu (2007) found that the optimal parameter when extracting the natural preservative from H. cordata using alcohol was a 1:15 solid/liquid ratio at 80°C for 10 h. The main strains which were inhibited by the volatile oils extracted from H. cordata in the food industry are shown in (CitationXiong et al., 2002; CitationTang et al., 2005). To determine the best preparation conditions for essential oil-β-cyclodextrin inclusion complex from H. cordata, an L9 (34) matrix was used to examine the effects of four factors, and the inclusion rate of each test was determined orthogonally. The best condition was oil-β-cyclodextrin- water = 1:8:80 (mL:g:mL), stirring for 1 h at 40°C. The resultant complex was stable and showed a high inclusion rate (CitationHe et al., 2005).
Table 4. Antimicrobial effect of the H. cordata extract.
Medicinal utilization of H. cordata
H. cordata has been used as TMC for thousands of years. In Chinese ancient medicine, infusion and decoction products of the H. cordata root and herb were used to prevent and cure diseases. In modern times the plant is gaining increasing popularity in the medical field for health promotion and adjuvant therapy. At present, the extracts and some products of H. cordata are used to treat respiratory problems, surgical diseases, gynecological diseases, infectious diseases, refractory hemoptysis, malignant pleural effusion and nephrotic syndrome, and so on (CitationZhou et al., 1999; CitationXu, 2002; CitationWu et al., 2006).
Future trends
H. cordata has been a time-honored TCM. Recently, clinical applications of H. cordata have increased. Although a number of studies have reported that H. cordata is effective in antiviral, antibacterial and anti-inflammatory functions, few studies have been conducted to examine the concentrations of pharmacological mechanisms for different diseases. Further attention has been paid to the side effects of the H. cordata. The results of some studies show H. cordata is available for several diseases afflicting mankind. Therefore, future studies will focus on the pharmacological components and functional mechanism of H. cordata.
H. cordata contains abundant nutritional and active ingredients, including volatile oils, flavonoids and water soluble polysaccharides. However, the products of H. cordata have not been widely accepted by consumers, and the application and development of potential products are lagging behind. In view of this, further research related to H. cordata utilization would not only be a scientific challenge but also an interesting economic pursuit.
With the increasing demand for the products of H. cordata, cultivated plants are gradually appearing. It is time for a series of good manufacturing practices to be mapped out to utilize the high-yield, environmentally friendly, high-quality resource of H. cordata.
Acknowledgements
This project was supported by National Key Technology R&D Program 2006BAD27B04.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ansari SH, Ali M, Qadry JS (1993): Essential oils of Pistacia integerrimagalls and their effect on the central nervous system. Int J Pharmacog 31: 89–89.
- Barmba D, Bessiere JM, Marion C (1993): Essential oil of Eupatorium odoratum. Planta Med 2: 184–184.
- Carlo GD, Mascolo N, Izzo AA, Capasso F (1999): Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci 65: 337–353.
- Chang JS, Chiang LC, Chen CC, Liu LT, Wang KC, Lin CC (2001): Atileukemic activity of Bidenspilosa l. var. minor (Blume) Sherff and H. cordata. Am J Chin Med 29: 303–312.
- Chen C, Huang H, Wang LH, Liu JY (2002): A study of the utilization status and trends of H. cordata. Crop Res 5: 262–265.
- Chen YY, Liu JF, Chen CM, Chao PY, Chang TJ (2003): A study of the antioxidative and antimutagenic effects of H. cordata using an oxidized frying oil-fed model. J Nutr Sci Vitaminol 49: 327–333.
- Chiang LC, Chang JS, Chen CC, Ng LT, Lin CC (2003): Anti-herpes simplex virus activity of Bidens pilosa and Houttuynia cordata. Am J Chin Med 31: 355–362.
- Chen L, Wu WZ, Heng YL (2004): HPLC analysis on dissociative amino acids of Houttuynia. Amino Acids Biot Resour 1: 20–22.
- Cao H, Wang SY (2005): The chemical constituent of H. cordata and the prospect of development. J Sountheast Guizhou Natl Teach Coll 23: 20–22.
- Chen GH, Cheng C (2005): Study on the microwave-assisted complex extraction technology of flavonoid and polysaccharide from Houttuynia cordata Thunb. J Hubei Inst Nationalities 23: 297–299.
- Chen SH, Tang YH, Zhou RB, He YS, Chen CF (2005): The volatile oils extract of H. cordata from different collection periods and the determination of ingredients. Chin Traditional Pat Med 27: 1333–1335.
- Cheng C, Li W (2006): Antioxidation of several plants’ water-soluble polysaccharides in vitro. Sci Technol Food Ind 27: 63–65.
- EI-TE, Eamauda MSM, Azzam AS (1994): Chemical composition and biological activity of the essential oil of Tagetes evecta L. cultivated in Egypt. Bull Fac Pharm 1: 113–113.
- Fu M, Wu XJ, Chen CG (2006): The quantitative analysis of nutritive composition from H. cordata. J Huaihua Univ 25: 70–71.
- Gong MZ (2003): Preparation of the health tea drink for the Herba Houttuyniae. Food Sci Technol 5: 75–77.
- Gurib-Fakim A (2006): Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med 27: 1–93.
- Guo YH, Xu YJ (2007): Extraction and purification of Houttuynia cordata flavonoids and identification of flavonoids type. Food Sci 28: 287–291.
- Hayashi K, Kamiya M, Hayashi T (1995): Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus and HIV. Planta Med 61: 237–241.
- Han XJ (2003): The efficacy and cultivation of H. cordata as an edible medicine. Plants 33: 41–41.
- He R, Hou SX, Guo DD (2005): Preparation technology for Houttuynia cordata essential oil-β-cyclodextrin inclusion complex. Cent South Pharm 3: 331–333.
- Illes V, Daood HG, Perneczki S, Szokonya L, Then M (2000): Fluids. J Supercrit 17: 177–177.
- Kwon KB, Kim EK, Shin BC, Seo EA, Yang JY, Ryu DG (2003): Herba houttuyniae extract induces apoptotic death of human promyelocytic leukemia cells via caspase activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Exp Mol Med 35: 91–97.
- Liang HX (1995): On the evolution and distribution in Saururaceae. Acta Bot Yunnan 17: 255–267.
- Li S, Yu QH, Zhang JS (1998): The anti-inflammatory and analgesic affect of houttuyninum. J Shenyang Pharm Univ 15: 272–274.
- Li W, Wang SJ, Yan M, Fu JH, Xie HX (1999): Observation of the inhibitory effect of extracts from H. cordata on IFVA, IFVB, MPV. Lit & Inf Prev Med 5: 347–348.
- Li SX (2001): Comprehension and suggestion on the Species Listed both as Foods and Drugs. Chin J Nat Med 3: 232–240.
- Li ZM, Dong R, Sun H (2004): Studies on some wild vegetable from central Yunnan. J Yunnan Normal Univ 22: 46–51.
- Li GZ, Chai OH, Lee MS, Han EH, Kim HT, Song CH (2005): Inhibitory effects of Houttuynia cordata water extracts on anaphylactic reaction and mast cell activation. Biol Pharm Bull 28: 1864–1868.
- Liang MM, Qi ML, Zhang CB, Zhou S, Fu RN, Huang JX (2005): Gas chromatography-mass spectrometry analysis of volatile compounds from H. cordata after extraction by solid-phase microextraction, flash evaporation and steam distillation. Anal Chim Acta 531: 97–104.
- Lu HM, Liang YZ, Yi LZ, Wu XJ (2006b): Anti-inflammatory effect of Houttuynia cordata injection. J Ethnopharmacol 104: 245–249.
- Lu HM, Wu XJ, Liang YZ, Zhang J (2006a): Variation in chemical composition and antibacterial activities of essential oils from two species of Houttuynia Thunb. Chem Pharm Bull (Tokyo) 7: 936–940.
- Li SH, Wu XJ, Yu JP, Hu MZ (2006): Study on extraction process of flavonoids from H. cordata leaves with supersonic extraction. Food Sci 27: 270–272.
- Liu SP, Xue YH (2006): Compounding H. cordata and Pueraria lobata to healthy beverage. Food Res Dev 27: 106–109.
- Li YQ, Fang L, Zhang KC (2007): Structure and bioactivities of a galactose rich extracellular polysaccharide from submergedly cultured Ganoderma lucidum. Carbohyd Polym 68: 323–328.
- Lukman S, He Y, Hui SC (2007): Computational methods for traditional Chinese medicine: A survey. Comput Meth Prog Biomed 88: 283–294.
- Miyazawa M, Shimamura H, Nakamura S (1996): Antimutagenic activity of (+)-β-eudesmol and paenonol from Dioscorea japonica. J Agric Food Chem 7: 1647–1647.
- Miyazawa M, Shimamura H, Kameoka H (1997): Antimutagenic activity of giganotol from Dendrobium nobile. J Agric Food Chem 8: 2849–2849.
- Meng J, Dong XP, Jiang ZH, Liang SX, Zhao ZZ (2006): Study on chemical constituents of flavonoids in the fresh herb of Houttuynia cordata. China J Chinese Materia Medica 31: 1335–1337.
- Qi YC, Hu C, Tian GZ, Li RG (2001): Comparison analysis of nutritive composition from the stem and leaf of Houttuynia cordata. Spec Wild Econ Anim Plant Res 24: 45–46.
- Qi ML, Ge XL, Liang MM, Fu RM (2004): Flash gas chromatography for analysis of volatile compounds from H. cordata. Anal Chim Acta 527: 69–72.
- Ren YC, Zhon YG, Lin WJ, Ye HZ (1998): Preparation of nutritional liquid of the cordata Houttuynia. Food and Mach 1: 13–13.
- Ren YL, Zhou YW, Ye YH (2004): Chemical components of Lactuca and their bioactivites. Acta Pharm Sin 11: 954–960.
- Sharma RN, Tere V, Pawar P (1992): Toxic effects of some plant oils and their common constituents on the psyllid pest, Heteropsylla cubana (Homoptera: psyllidae) of social for estry tree leucaephala. Appl Entomol Zool 2: 285–285.
- Shao L, Yu QH, Wu HY, Li X (1999): The effects of houttuyninum on immunologic function of splenectomized mice. J Shenyang Pharm Univ 16: 209–211.
- Sheng Q, Ren YC, Zhou YG (1999): Determination of flavones contents from Houttuynia cordata. Sci Technol Food Ind 20: 64–65.
- Schepetkin IA, Quinn MT (2006): Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. International Immunopharmacol 6: 317–333.
- Tanaka S, Akimoto A, Tamb Y (1996): Volatile antiallengic principles from a traditional herbal prescriplion of kampo medicine. Phytother Res 3: 238–238.
- Tang BX, Duan LY, Wang FY, Zhao LZ, Yu YG, Li SX (2005): Extraction and antibiotic action of volatile oil from Houttuynia cordata. J Shaoyang Univ 2: 80–81.
- Wu SQ, Li XS, Zhou JJ (2000): Determination of amino acids and other nutritious component in Osmunda japonica Thanb and H. cordata. Amino Acids Biotic Resour 3: 65–67.
- Wu TX, Tang QL (2001): Studies on H. cordata, pumpkin and Grossularia burejensis Berger acetic fermentation beverage. Mach Cereals Oil Food Proc 5: 36–37.
- Wu PY, Xu LY, Tao JS (2006): Research progress of Herba Houttuyniae. SH J TCM 40: 62–64.
- Xiao M, Liu B, Zhao RZ (2003): Study on assaying minerals contents in Houttuynia cordata Thunb. and the processing of its beverage. Food Sci 24: 73–76.
- Xiong DS, Xi ZX, Deng YW (2002): Studied of the antimicrobial effect of the extracts of Houttuynia cordata. J Changde Teach Univ 14: 59–60.
- Xu CY (2002): Observing the effect in treating infant acute upper respiratory tract infection by intravenous drip with houttuynine sodium injection. Hebei Med 8: 326–327.
- Xu CJ, Liang YZ, Chaub FT (2005): Identification of essential components of Houttuynia cordata by gas chromatography/mass spectrometry and the integrated chemometric approach. Talanta 68: 108–115.
- Xu XQ, Ye HZ, Wang W, Yu LS, Chen GN (2006): Determination of flavonoids in H. cordata and Saururus chinensis (Lour.) Bail. by capillary electrophoresis with electrochemical detection. Talanta 68: 759–764.
- Xu Y (2007): On the method of extracting natural preservative in H. cordata.and its bacteriostasis. J Hubei Univ Technol 22: 75–77.
- Yang JK (2003): Saururaceae, a new family of plants in Hebei. Hebei J Forestry Orchard Res 18: 336–337.
- You JM (2006): Study on separation total flavones from H. cordata with macroporous exchange resin. Mod Food Sci Technol 21: 66–68.
- Zhou X, Chen JX, Li GM (1999): The observation of therapy on treating herpes zoster with H. cordata injection. Acta Univ Sci Med 24: 408–409.
- Zhang Q, Qin LK, Yuan Y, Tu H, Yang XJ (2000): Study on extraction and assessment of polysaccharide hydrolysates of H. cordata. Food Sci 21: 49–50.
- Zheng YX, Li NF (2002): The development and utilization of Houttuygnia. J Changjing Vegetables 12: 8–9.
- Zeng Z, Shi JG, Zeng HP, Lai WL (2003): Application of organic mass spectrometry in studies on Houttuynia cordata, a Traditional Chinese Medicine. Chin J Anal Chem 31: 399–404.
- Zhan XJ, Zhang L, Zhou Z, Cheng YL (2006): The preparation of jerky-beef of Houttuygnia cordata Thunb. Modern Food Sci Technol 4: 22–24.
- Zhang Y, Li SF, Wu XW (2007): Pressurized liquid extraction of flavonoids from Houttuynia cordata Thunb. Sep Purif Technol doi:10.1016/j.seppur.2007.04.010.
- Zhang X, Wan XX, He SH (2005): Studies on compound recipe H. cordata tea. J Branch Campus First Military Med 28: 132–133.
- Zhou F (2006b): Study on the processing technology of soft-packing flavoured H. cordata. Sichuan Food Fermn 42: 20–22.
- Zhou GH, Yu HZ, Yu YH (2006): Study on bacteriostatic action in different parts of Houttuynia cordata. Hunan Forest Sci & Technol 33: 38–40.
- Zhou Z (2006a): Study on the processing technology for Houttuynia cordata’s convenient food. Sci Technol Food Ind 27: 119–121.
- Zhou Z (2007): Studies on the processing of fried food from Houttuynia cordata Thunb. and its critical control point. J Hubei Inst Natl 25: 184–187.