Abstract
A thorough comparative analysis of cytotoxic effect of an aqueous cinnamon extract (ACE) from the bark of Cinnamomum zeylanicum L. (Lauraceae) with that of commercially available cinnamaldehyde was performed on various human as well as mouse cell lines and primary cells. The aqueous cinnamon extract (ACE) proved to be more cytotoxic to cancerous cells at concentrations just above 0.16 mg/mL (containing 1.28 μM cinnamaldehyde) around which the commercial cinnamaldehyde (1.6 μM) had no cytotoxic effect. At a critical concentration of 1.28 mg/mL (containing 10.24 μM cinnamaldehyde), ACE treatment resulted in 35-85% growth inhibition of the majority of the cancerous cells, whereas at a similar concentration (10 μM) commercial cinnamaldehyde treatment resulted in 30% growth inhibition of only SK-N-MC cells with no effect on other cell lines. These results suggest that ACE had a significant inhibitory effect on the majority of cancer cells and thus may prove to be a chemotherapeutic agent.
Introduction
Cancer continues to represent the largest cause of mortality worldwide. An important goal of cancer chemotherapy is to selectively destroy the tumor cells without damaging the normal cells. Currently, the plants, vegetables, herbs and spices used in folk and traditional medicine have been accepted as sources possessing anticancer potential (CitationAbdullaev, 2002). The influence of dietary factors on cancer risk has been indicated by epidemiological and animal model studies (CitationWattenberg, 1992). A wide variety of naturally occurring substances from plant food including spices have been shown to offer protection from carcinogenic exposure (CitationBanerjee et al., 2006).
The derivatives of cinnamaldehyde, the active component of the spice cinnamon, have been shown to possess various biological activities (CitationJeong et al., 2003) including anti-angiogenic and immunomodulatory activity. Cinnamaldehyde has been shown to inhibit proliferation of several human cancer cell lines including breast, leukemia, ovarian, and lung tumor cells (CitationLee et al., 1999). Cinnamomum zeylanicum L. (Lauraceae) is reported to have originated in Sri Lanka and the Malabar coast of India (CitationRadhakrishnan et al., 1992). This plant has been used for many purposes since ancient times and its leaves and bark are used in various food applications (CitationJirovetz et al., 1997). Recently it has been shown that essential oil of Cinnamomum zeylanicum from Cameroon possesses antiradical and antifungal activities against some common fungi causing spoilage of stored food products (CitationDongmo et al., 2007).
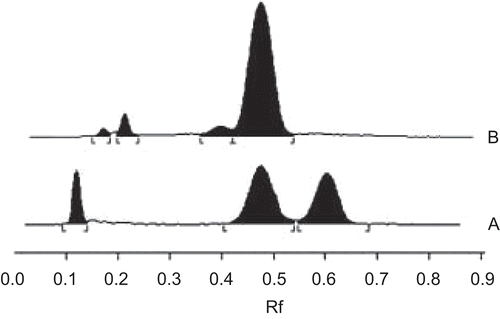
Figure 1. HPTLC chromatograms of (A) standard mixture of piperine, cinnamaldehyde and eugenol; (B) aqueous cinnamon extract (ACE). The amount of cinnamaldehyde present in ACE was determined from the calibration curve obtained by plotting the concentration of standard against the peak area of test sample (ACE). The quantity of cinnamaldehyde in ACE was found to be around 0.25% w/v. Thus, 50 mg/mL stock solution of ACE contained 400 μM cinnamaldehyde content. (B) Calibration curve for quantification of analytes from ACE.
Here we have used the aqueous extract of Cinnamomum zeylanicum bark to study its cytotoxic activity on an array of cell lines and primary cells that has not been reported untill now to the best of our knowledge. A comparative study of cytotoxic effect was carried out for the first time with that of commercially available cinnamaldehyde to correlate the potential cytotoxicity of ACE, which can be implicated as a chemotherapeutic nutraceutical.
Materials and methods
Materials
Tissue culture plasticware were purchased from BD Biosciences, CA, USA, Axygen Scientific Inc, CA, USA Axygen Corporations, India, and Nunc, Roskilde, Denmark. Dulbecco’s modified Eagle’s medium (DMEM) was obtained from HiMedia Laboratories, Mumbai, India. Penicillin and streptomycin were obtained from Gibco BRL, CA, USA. Fetal calf serum was purchased from Biological Industries Ltd, Kibbutz Beit Haemek, Israel and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylthiazolium bromide (MTT) was purchased from Sigma-Aldrich (St. Louis, MO). Cinnamaldehyde (98% purity) was obtained from the Central Drug House, New Delhi, India.
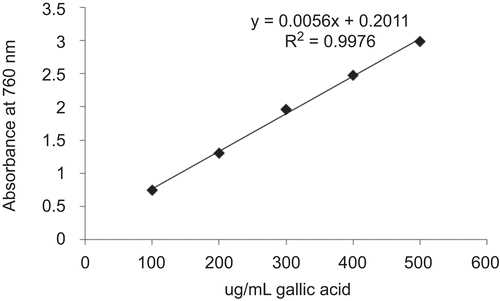
Figure 2. The amount of polyphenols present in ACE was determined from the calibration curve obtained by plotting the concentration of standard gallic acid equivalent against the test sample (ACE). The typical regression equation was y = 0.0056 x+ 0.2011 for polyphenols. The quantity of polyphenols in 50 mg/mL ACE was found to be 380.83 μg/mL or 0.76%. Standard plot obtained by plotting the concentration of gallic acid equivalent.
Plant materials and extract preparations
The bark of Cinnamomum zeylanicum L. (Lauraceae) was purchased from Ashwanikumar Herbo-Minerals & Aromatic Genuine Resources, Pune, Maharashtra, India. Voucher specimen number for Cinnamomum zeylanicum bark was 464. The Cinnamomum zeylanicum bark was authenticated at the Department of Botany, Agharkar Research Institute, Pune, India by Dr. A. M. Mujumdar, Head Plant Sciences Division. The bark was powdered and weighed to make a stock of 50 mg/mL solution in water which was subjected to 15 pounds pressure at 121°C for 20 min. The aqueous cinnamon extract (ACE) solution was centrifuged at 10,000 rpm for 10 min and the supernatant containing the extract was stored in aliquots at -80°C. Prior to dosing the cells, the extract was filter sterilized (pore size, 0.45 μm).
HPTLC analysis
The bark of Cinnamomum zeylanicum was purchased from an authentic source and its identity was further confirmed by detecting the marker molecule cinnamaldehyde in ACE (50 mg/mL stock solution) by HPTLC analysis as described previously (Gopu et al., 2007). In brief, the samples (standards and test material) were spotted as bands (8 mm width) with a Camag (Muttenz, Switzerland) microliter syringe onto a pre-coated aluminum-backed silica gel 60F-254 plate (20 × 10 cm; layer thickness 250 μm; Merck, Darmstadt, Germany) using a Camag Linomat IV automatic sample spotter. A constant application rate (0.1 μL/s) was employed and the space between the two bands was 6 mm. A reference standard stock solution containing 52.54 μg/mL of cinnamaldehyde (Central Drug House) of 98% purity, 533.2 μg/mL of eugenol (Loba Chemie, Mumbai, India) of 98% purity and 50 μg/mL of piperine (Natural Remedies, Bangalore, India) of 98.2% purity, was freshly prepared in methanol, and aliquots of 5 μL were applied to the TLC plate along with the test solution. The mobile phase consisted of petroleum ether:dichloromethane:formic acid (2:4:0.1, v/v/v). Linear ascending development was carried out in twin trough glass chambers saturated with the mobile phase. The optimum chamber saturation time for the mobile phase was 20 min at room temperature. The plates were developed up to 9 cm under chamber saturation conditions. Subsequent to development, TLC plates were dried in a current of air with the help of an air-dryer. A Camag model III TLC scanner with CATS 4.0 integration software was used. Densitometric scanning was performed in the absorbance mode at 290 nm with a slit dimension of 6 × 0.45 mm and a scanning speed of 10 mm/s. A deuterium lamp was used as source of radiation. The amount of cinnamaldehyde present in ACE was determined from the calibration curve obtained by plotting the concentration of standard against the peak area of test sample (ACE). The peak area versus concentration plots was found to be linear in the range of 52.54–735.56 ng/ spot for cinnamaldehyde with a correlation coeficient of 0.9985 ± 0.04. The typical regression equation was y = 38.269x + 499.14 for cinnamaldehyde. The quantity of cinnamaldehyde in ACE was found to be around 0.25% w/v. Thus, 50 mg/ mL stock solution of ACE contained 400 μM cinnamaldehyde content.
Detection of total polyphenol content in the extract
The total polyphenolic content of ACE was measured by the Folin-Ciocalteau method. Initially, 0.5 mL of 50 mg/mL ACE was mixed with 0.5 mL of the Folin-Ciocalteau reagent and 0.5 mL of 10% Na2CO3. After 1 h incubation at room temperature, the blue color formed due to the polyphenols present in the extract was measured at 760 nm using UV spectrophotometer and they were expressed as μg/mL of gallic acid equivalent (CitationKumazawa et al., 2004). The amount of polyphenols present in ACE was determined from the calibration curve obtained by plotting the concentration of standard against the test sample (ACE). The quantity of polyphenols in 50 mg/mL ACE was found to be around 380.83 μg/mL or 0.76%.
Cell culture
The cell lines used in the study were: RAW 264.7 (murine macrophage), Neuro 2a (mouse neuroblastoma), MG63 (human osteosarcoma), HT1080 (human fibrosarcoma), L929 (mouse connective tissue), SiHa (human cervix carcinoma), A431 (human skin carcinoma), SK-N-MC (human neuroblastoma), which were obtained from the National Centre for Cell Science (NCCS), Pune, India. Mouse primary fibroblast and hepatocytes were isolated and cultured in-house. All the cells were grown in DMEM containing 2 mM l-glutamine and supplemented with 10% fetal calf serum and 100 U/mL of penicillin and streptomycin sulfate. The cells were incubated in a humidified 5% CO2 incubator at 37°C.
MTT assay
Cell viability was determined by MTT [3-(4,5- dimethylthiazol-2-yl)-2,5-diphenylthiazolium bromide] assay. RAW 264.7 cells were seeded at 5 × 105/ mL density and other cell lines/primary cells at 1 × 105/ mL in 96-well plates. An untreated group was kept as a control in all the cell lines/primary cells used. The aqueous cinnamon extract (ACE) was added at the following concentrations: 0.04, 0.08, 0.16, 0.32, 0.64, and 1.28 mg/mL, in each well in triplicate and the plates were incubated for 24 h at 37°C in 5% CO2 incubator. Similarly, cells were treated with commercial cinnamaldehyde diluted in DMSO (CitationYoun et al., 2008) at different concentrations: 0.8, 1.6, 3.2, 5, 10, 20, 40, 80, 160, and 320 μM. The experiments were repeated at least four times with all the cells. The MTT solution (5 mg/mL) was added to each well, and the cells were cultured for another 4 h at 37°C in 5% CO2 incubator. The formazan crystals [1-(4,5-dimethyl thiazol-2-yl)-3,5-diphenyl formazan] formed were dissolved by the addition of 90 μL of SDS-DMF (20% SDS in 50% DMF) and mixed at room temperature. After 15 min, the level of colored formazan derivative was determined by measuring optical density (OD) with the ELISA microplate reader (Biorad, Hercules, CA) at 570 nm (OD570-630nm). All assays were performed in triplicate and the mean OD value of each triplicate (for different ACE concentrations) in all the cells used was expressed as a percentage of the mean OD of control cells. The percentage viability was calculated as:
Statistical analysis
All experiments were performed in triplicate and repeated at least four times and the data were presented as mean ± SD. Student’s t-test was performed on data showing comparative effect of ACE and commercial cinnamaldehyde on percentage cell survival at 10 μM concentration, with respective p-values for each cell line tabulated.
Results and discussion
Effect of aqueous cinnamon extract (ACE) from bark of C. zeylanicum on viability of various cell lines and primary cells
The inhibitory effect of ACE on the proliferation of various cell lines and primary cells was analyzed by MTT assay and the reading was compared with the respective control groups (untreated cells) that showed 100% survival. The concentration of cinnamaldehyde (μM) present in the ACE was determined by HPTLC analysis () and has also been shown in brackets against the respective mg/mL concentration in . It was found that 50 mg/mL stock solution of ACE contained 400 μM cinnamaldehyde content. The presence of polyphenols was confirmed by Folin-Ciocalteau method (). The quantity of polyphenols in 50 mg/ mL ACE was found to be around 380.83 μg/ mL (equivalent to 0.76%).
Table 1. Effect of different concentrations of aqueous cinnamon extract (ACE) on cell viability.
A dose-dependent decrease in the percentage survival of ACE-treated cells was observed as the concentration of ACE increased from 0.04 to 1.28 mg/mL for each cell line/primary cells used (). The anti-proliferative property of ACE was observed to start from 0.16 mg/ mL for MG63 and at/above 0.32 mg/ mL concentration for other cells. At the highest dose of 1.28 mg/mL, few cell lines such as HT1080, A431, SK-N-MC and SiHa exhibited around 50-75% decrease in cell viability. At the same dose, other cell lines (MG63 and Neuro2a) demonstrated 35% decrease in cell survival. On the other hand, murine connective tissue (L929) and macrophage (RAW 264.7) cell line showed around 80-100% survival whereas mouse primary fibroblasts and hepatocytes exhibited 63% and 84% viability, respectively. An interesting observation to note was that compared to the mouse primary fibroblasts (63% survival), percentage survival was less for both the fibrosarcoma cell lines (23% for A431 and 44% for HT1080). In any conventional chemotherapy, the chemotherapeutic drug doesn’t show cell specificity and as a result the normal cells also get killed along with the cancerous cells. But with ACE, the percentage survival of primary cells was more compared to the cancerous cells and thus could prove to be a promising chemotherapeutic nutraceutical.
Compared to other cell lines, MG63 and Neuro2a exhibited relatively less susceptibility at the higher dose suggesting that certain cell lines require higher doses of the ACE. But before selecting a higher dose it becomes essential to consider that the selected dose should not be over toxic to the primary cells.
Effect of commercial cinnamaldehyde on viability of various cell lines and primary cells
Cinnamaldehyde and its derivatives have been shown to possess anti-proliferative activity on various cancer cell lines (CitationLee et al., 1999). Thus, we compared the antiproliferative effect of commercial cinnamaldehyde with that of ACE from the bark of C. zeylanicum on various cell lines and primary cells. The cells were exposed to different concentrations of commercial cinnamaldehyde (0.8 to 320 μM) overnight at 37°C in 5% CO2 incubator followed by MTT assay.
depicts the effect of increasing concentration of cinnamaldehyde on different cell lines and primary cells. It was observed that the cells showed differential response in viability to various concentrations of cinnamaldehyde. Out of all the cells tested, SK-N-MC was the only cell line that showed a decrease in survival (around 30%) at a concentration of 10 μM cinnamaldehyde, whereas mouse hepatocytes and mouse primary fibroblast showed around 12-15% decrease in cell viability, respectively. At 20 μM cinnamaldehyde concentration, SiHa and L929 showed around 10-12% decrease in viability, respectively. At and above 40 μM, most of the cell lines (HT1080, A431 and Neuro2a) showed a dose-dependent decrease in cell survival. On the other hand, MG63 cells showed a decrease in viability (around 70%) at a much higher dose of cinnamaldehyde (320 μM) at which the survival of other cells varied between 1% and 9% as observed in . These results suggest that although certain cancers such as osteosarcoma required higher concentrations of cinnamaldehyde to induce cytotoxicity, this concentration would be toxic to the primary cells as well.
An interesting observation to note here is that higher concentrations (above 20 μM) of cinnamaldehyde are required to inhibit the proliferation of the majority of the cancerous cells which is not the case with ACE wherein the cells are inhibited at concentrations just above 1.28 μM. On comparing the cytotoxicity data of ACE at 1.28 mg/mL (containing 10.24 μM cinnamaldehyde) with that of commercial cinnamaldehyde at 10 μM concentration (), we observed that ACE was significantly more cytotoxic compared to the commercial cinnamaldehyde. ACE treatment resulted in 35-80% growth inhibition of the majority of cancerous cells, whereas at 10 μM commercial cinnamaldehyde exposure, SK-N-MC cells showed 30%, whereas primary fibroblast showed only 15% growth inhibition. Other cells required higher doses of cinnamaldehyde to show the cytotoxic effect. The p values for the cell lines that show a statistically significant difference in survival upon ACE compared to cinnamaldehyde treatment at 10 μM concentration are shown in except for RAW264.7 and mouse hepatocytes that did not show any significant difference in cell survival. Moreover, with ACE, L929 cells and mouse primary fibroblasts demonstrated only 20-35% growth inhibition and as a result exhibited 65-80% cell survival at a dose that was far more toxic to the cancerous cells.
Table 2. Effect of different concentrations of commercial cinnamaldehyde on cell viability.
Table 3. Comparative analysis of cytotoxicity of ACE with that of commercial cinnamaldehyde.
Reports from both epidemiological and experimental studies suggest that polyphenols present in plants prevent the development of various cancers (CitationPark & Pezzuto, 2002), probably through their ability to function as antioxidants. Recently, it has been shown that water-soluble polymeric polyphenols from cinnamon inhibit proliferation and alter cell cycle through their potential to interact with phosphorylation/dephosphorylation signaling activities (CitationSchoene et al., 2005). The increased cytotoxic activity of ACE could be explained due to the presence of polyphenolic compounds in the extract. Thus, polyphenols may be acting synergistically with the cinnamaldehyde present in the aqueous extract of cinnamon (ACE) and thereby inducing the increased cytotoxic activity compared to the commercial cinnamaldehyde. These findings suggest the potential use of ACE as an effective chemotherapeutic nutraceutical.
Acknowledgements
We thank Dr. P. K. Ranjekar, Director, Interactive Research School for Health Affairs (IRSHA) for allowing us to complete this work.
Declaration of interest: We also thank IRSHA, Bharati Vidyapeeth University for funding this work. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abdullaev FI (2002): Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.). Exp Biol Med 227: 20–25.
- Banerjee S, Panda CK, Das S (2006): Clove (Syzygium aromaticum L.), a potential chemopreventive agent for lung cancer. Carcinogenesis 27: 1645–1654.
- Dongmo PMJ, Tatsadjieu LN, Tchoumbougnang F, Sameza ML, Dongmo BN, Zollo PHA, Menut C (2007): Chemical composition, antiradical and antifungal activities of essential oil of the leaves of Cinnamomum zeylanicum Blume from Cameroon. Nat Prod Commun 2: 1287–1290.
- Gopu CL, Aher S, Mehta H, Paradkar AR, Mahadik KR (2008): Simultaneous determination of cinnamaldehyde, eugenol and piperine by HPTLC densitometric method. Phytochemical Analysis 19: 116–121.
- Jirovetz L, Buchbauer G, Ngassoum MB (1997): GC/MS-analysis of essential oils from Cameroon plants used as spices in local foodstuff. Recent Res Dev Agric Food Chem 1: 241–255.
- Jeong HW, Han DC, Son KH, Han MY, Lim JS, Ha JH, Lee CW, Kim HM, Kim HC, Kwon BM (2003): Antitumor effect of the cinnamaldehyde derivative CB403 through the arrest of cell cycle progression in the G2/M phase. Biochem Pharmacol 65: 1343–1350.
- Kumazawa S, Hamasaka T, Nakayama T (2004): Antioxidant activity of propolis of various geographic origins. Food Chem. 84: 329–339.
- Lee CW, Hong DH, Han SB, Park SH, Kim HK, Kwon BM, Kim HM (1999): Inhibition of human tumor growth by 20-hydroxy- and 20-benzoyloxycinnamaldehydes. Planta Med 65: 263–266.
- Schoene NW, Kelly MA, Polansky MM, Anderson RA (2005): Water-soluble polymeric polyphenols from cinnamon inhibit proliferation and alter cell cycle distribution patterns of hematologic tumor cell lines. Cancer Lett 230: 134–140.
- Park EJ, Pezzuto JM (2002): Botanicals in cancer chemoprevention. Cancer Metastasis Rev 21: 231–255.
- Radhakrishnan VV, Madhusoodnan KJ, Kuruvilla KM (1992): Cinnamon – The spicy bark. Spice India 5(4): 12–13.
- Wattenberg LW (1992): Inhibition of carcinogenesis by minor dietary constituents. Cancer Res 52: 2085–2091.
- Youn HS, Lee JK, Choi YJ, Saitoh SI, Miyake K, Hwang DH, Lee JY (2008): Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem Pharmacol 75: 494–502.