Abstract
Long-term treatment with haloperidol, a typical neuroleptic, induces neurodegeneration caused by excitotoxicity and oxidative stress, which play an important role in the development of orofacial dyskinesia. In the present investigation, an attempt has been made to examine the effect of a concomitant treatment of methanol extract of Morus alba Linn. (Moraceae) leaves (100–300 mg/kg, i.p.) and haloperidol (1 mg/kg, i.p.) on an animal model of tardive dyskinesia. Rats were treated for 21 days with haloperidol and Morus alba extract; vacuous chewing movements and tongue protrusions were counted. The extract attenuated the increase in vacuous chewing movements and tongue protrusions induced by haloperidol, which were quantified on day 22. The extract showed a marked effect on behavioral parameters altered by haloperidol treatment. Similar treatment with extract attenuated haloperidol-induced lipid peroxidation and nitrite and normalized superoxide dismutase, catalase, and protein in comparison to the control group. The results suggest a protective effect of Morus alba extract against haloperidol-induced orofacial dyskinesia and oxidative stress.
Introduction
The term “tardive dyskinesia” was first proposed in 1960 by Uhrbrand and Faurbye. The symptoms referred to as tardive dyskinesia (TD) attracted special attention in connection with the introduction of neuroleptics in the treatment of schizophrenia (CitationWolfarth & Ossowska, 1989). Haloperidol is a typical neuroleptic extensively used in the treatment of schizophrenia and other affective disorders, which unfortunately often causes distressing extrapyramidal side effects. The adverse reactions comprise a variety of movement disorders including TD, which occurs in 20–40% of the patient population taking chronic neuroleptic medication of the classic type; this is a major limitation of neuroleptic therapy (CitationNaidu et al., 2003).
The pathophysiological basis of TD remains unclear, but several lines of evidence suggest that the neuronal changes in basal ganglia produced by increased oxidative stress, products of lipid peroxidation, and glutamate excitotoxicity may play a role (CitationBishnoi et al., 2008). In rats treated chronically with antipsychotics, the display of spontaneous oral movements such as vacuous chewing movements is often considered as a valid animal model of TD (CitationIkeda et al., 1999). The involuntary choreoathetotic movements involve predominantly the muscles of the face and tongue, but other parts of the body may also be affected. Aging and brain damage are consistently found to be risk factors for developing TD (CitationAndreassen & Jorgensen, 1994).
Chronic treatment with neuroleptic medication increases free radical production and oxidative stress. A role for increased reactive oxygen species and oxidative stress in the etiology of neuroleptic-induced TD has been proposed. The administration of a single dose of haloperidol to mice led to an increase in oxidized glutathione levels in the striatum, indicating generation of oxidative stress by the drug. It has been reported that rats with vacuous chewing movements had significantly higher thiobarbituric acid reactive substances (TBARS) in the striatum, suggesting increased lipid peroxidation and free radical production in these animals. The activity of antioxidant defense enzymes such as superoxide dismutase (SOD) and catalase decreased due to chronic use of neuroleptics. Free radicals are thought to play a role in the aging process, and age is one of the risk factors for the development of TD. All these accumulating data strongly support the free radical hypothesis of TD (CitationNaidu et al., 2002).
Morus alba Linn. is one of the best known and most widely distributed trees or shrubs of the family Moraceae, and is extensively cultivated throughout the plains of India. It is locally known as mulberry. Morus species are an important food commodity for silkworms, which have received enormous attention in the last few years (CitationAnonymous, 1956). A number of medicinal properties have been ascribed to various parts of this highly esteemed tree. The leaves of the mulberry tree have been used in traditional Chinese and Indian medicine as an astringent, anthelmintic, antibacterial, purgative, diaphoretic, diuretic, analgesic, antiasthmatic, antirheumatic, antitussive, emolient, expectorant, hepatoprotective, hypoglycemic, and brain tonic (CitationNadkarni, 1976). Besides the above, they have shown antiviral and antimicrobial activity (CitationDu et al., 2003). The plant has been extensively studied for its hypotensive, hypoglycemic, neuroprotective (CitationFukai et al., 1985; CitationSingab et al., 2005; CitationTong et al., 2006), hepatoprotective (CitationHyuncheol et al., 2002), hypouricemic (CitationCheyl, 2006), cardioprotective (CitationByambaa et al., 2005), and anxiolytic (CitationYadav et al., 2008) effects. On the other hand, the antioxidant potency of some phenolic compounds from Morus alba has been reported (CitationFukai et al., 2003). Naturally occurring flavonoids, especially those of Morus alba, have shown antioxidant activity in different model systems (CitationFukai et al., 1985; CitationKim et al., 1999; CitationByambaa et al., 2005; CitationEl-Beshbishy et al., 2006). The leaves are a very good source of ascorbic acid, of which over 90% is present in reduced form. They also contain carotene, vitamin B1, folic acid, folinic acid, isoquercetin, quercetin, tannins, flavonoids, and saponins, which act as a good source of natural antioxidants (CitationAnonymous, 1956), providing a way to study the molecular mechanisms underlying the antioxidant role of the mulberry.
In this study, we have investigated the effect of the methanol extract of Morus alba leaves (MAE) on haloperidol-induced orofacial dyskinesia and measures of oxidative stress in rats.
Materials and methods
Plant material
The fresh leaves of the plant were collected in the month of October (2007) from the local area in Nashik, India and authenticated by P. S. N. Rao (Director, Botanical Survey of India, Pune). A voucher specimen of the plant has been deposited at the Botanical Survey of India, Pune (Voucher Specimen No. NVMA2). The leaves were shade-dried and reduced to coarse powder. The powdered plant material (1 kg) was defatted using petroleum ether (60–80°C) by Soxhlet apparatus and then extracted by methanol for 72 h. The extract obtained was filtered and concentrated under reduced pressure. The yield of methanol extract of Morus alba leaves was found to be 4.1%, w/w. The dried extract was dissolved in distilled water and administered intraperitoneally (i.p.).
Animals
Male Wistar strain rats (170–220 g) were used for the study. Animals were housed in colony cages and maintained under standard laboratory environmental conditions: temperature 25 ± 2°C, 12 h light/12 h dark cycle, and 50 ± 5% relative humidity, with access to food and water ad libitum. Animals were acclimatized to laboratory conditions before the test. Each group consisted of five animals. All experiments were carried out during the light period (08:00–16:00 h). The studies were carried out in accordance with the guidelines given by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi (India). The Institutional Animal Ethics Committee of N.D.M.V.P.S College of Pharmacy, Nashik, approved the protocol of the study (IAEC/2007/01).
Drugs and chemicals
Haloperidol injection (RPG Life Sciences Ltd., Ankaleshwar, India) was diluted with distilled water. All the chemicals used were of analytical grade and purchased from standard manufacturers.
Phytochemical screening
Screening for the presence of phenolic compounds, tannins, flavonoids, alkaloids, glycosides, triterpenes, sterols, anthocyanins, and anthroquinones was carried out using methods previously described (CitationKokate, 1994; CitationTrease & Evans, 1997).
Treatment schedule
Animals were divided into six groups (n = 5 for each group): group I, vehicle for MAE (distilled water); group II, haloperidol (1 mg/kg, i.p.) + vehicle for MAE; group III, haloperidol (1 mg/kg, i.p.) + MAE (100 mg/kg, i.p.); group IV, haloperidol (1 mg/kg, i.p.) + MAE (200 mg/kg, i.p.); group V, haloperidol (1 mg/kg, i.p.) + MAE (300 mg/kg, i.p.); group VI, only MAE (300 mg/kg, i.p.).
Induction of orofacial dyskinesia
Haloperidol (1 mg/kg, i.p.) was administered chronically to rats for a period of 21 days to induce orofacial dyskinesia. Behavioral assessments were carried out 24 h after administration of the last dose of haloperidol (CitationNaidu et al., 2003).
Behavioral testing
To quantify the occurrence of oral dyskinesia on the test day, rats were placed individually into a small Plexiglas observation cage (30 × 20 × 20 cm) to enable vacuous chewing movements (VCMs) and tongue protrusion frequencies to be scored. Animals were allowed 10 min to acclimatize to the observation cage before behavioral assessments were performed. Mirrors were placed under the floor and behind the back wall of the cage to permit observation of oral dyskinesia when the animal was facing away from the observer. The VCMs and tongue protrusions were defined as single mouth openings in the vertical plane not directed toward physical material, and visible extensions of the tongue outside of the mouth, respectively. If VCMs or tongue protrusions occurred during a period of grooming, they were not taken into account. The behavioral parameters of oral dyskinesia were measured continuously for a period of 5 min. In all the experiments, the observer was blind to the identity of the animals (CitationNaidu et al., 2003).
Biochemical analysis
Dissection and homogenization
On the 22nd day of haloperidol treatment, immediately after behavioral assessments, the animals were killed by decapitation. The brain was removed, rinsed with isotonic saline, and weighed. A 10% (w/v) tissue homogenate was prepared in 0.1 M phosphate buffer (pH 7.4). The post-nuclear fraction for catalase assay was obtained by centrifugation (Remi C-30; Remi Industries Ltd., Mumbai, India) of the homogenate at 1000g for 20 min at 4°C; for other enzyme assays, centrifugation was at 12,000g for 60 min at 4°C. A Shimadzu-160A spectrophotometer was used for subsequent assays (CitationNaidu et al., 2003).
Catalase activity (CAT)
Catalase activity was assessed by the method of CitationLuck (1971), where the breakdown of H2O2 is measured at 240 nm. Briefly, the assay mixture consisted of 3 mL of H2O2 phosphate buffer (0.0125 M H2O2) and 0.05 mL of supernatant of brain homogenate (10%), and the change in absorbance was measured at 240 nm. The enzyme activity was calculated using the millimolar extension coefficient of H2O2 (0.07). The results are expressed as micromoles of H2O2 decomposed per minute per milligram of protein.
Superoxide dismutase activity (SOD)
Superoxide dismutase activity was assayed according to the method of CitationKono (1978), wherein the reduction of nitroblue tetrazolium chloride (NBT) inhibited by superoxide dismutase is measured at 560 nm spectrophotometrically. Briefly, the reaction was initiated by the addition of hydroxylamine hydrochloride to the reaction mixture containing NBT and post-nuclear fraction of brain homogenate. The results are expressed as units per milligram of protein, with one unit of enzyme defined as the amount of SOD required to inhibit the rate of reaction by 50%.
Lipid peroxidation assay (LPO)
The quantitative measurement of lipid peroxidation in the brain was done by the method of CitationWills (1966). The amount of malondialdehyde (MDA) formed was measured by reaction with thiobarbituric acid at 532 nm. The results are expressed as nanomoles of MDA per milligram of protein, using the molar extension coefficient of the chromophore (1.56 × 105 M−1 cm−1).
Nitrite estimation (NO)
The accumulation of nitrite in the supernatant, an indicator of the production of nitric oxide (NO), was determined with a colorimetric assay using Griess reagent [0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, 1% sulfanilamide, and 2.5% phosphoric acid] according to CitationGreen et al. (1982). Equal volumes of supernatant and Griess reagent were mixed, the mixture was incubated for 10 min at room temperature in the dark, and the absorbance at 543 nm was determined spectrophotometrically. The concentration of nitrite in the supernatant was determined from a sodium nitrite standard curve and is expressed as micromoles of nitrite per milliliter of homogenate.
Protein estimation
The protein content was measured according to the method of CitationLowry et al. (1951), using bovine serum albumin as standard and expressed as micrograms of protein per milligram of tissue.
Statistical analysis
Results are expressed as mean ± SEM, and the statistical analysis of data was done using one-way analysis of variance (ANOVA) followed by Dunnett’s test. A probability level of less than 0.05 was considered statistically significant.
Results
Phytochemical screening
The phytochemical screening of MAE revealed the presence of phenolic compounds, flavonoids, tannins, anthocyanins, anthroquinones, sterols, alkoloids, and saponins.
Behavioral assessment
Assessment of orofacial dyskinesia
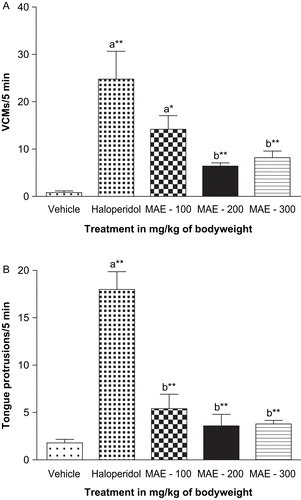
The frequencies of VCMs and tongue protrusions in rats were significantly increased after chronic treatment with haloperidol (1 mg/kg), compared to vehicle-treated controls. Chronic concomitant administration of MAE (100– 300 mg/kg) for a period of 21 days along with haloperidol significantly attenuated the haloperidol-induced VCMs and tongue protrusions dose-dependently (p < 0.05, p < 0.01). MAE alone did not induce any VCMs or tongue protrusions ().
Figure 1. Effect of coadministration of Morus alba leaf extract (MAE) on chronic haloperidol-induced (a) vacuous chewing movements (VCMs) and (b) tongue protrusions. Each column represents mean ± SEM (n = 5). aCompared with vehicle-treated group; bcompared with haloperidol-treated group; *p < 0.05; **p < 0.01 (one-way ANOVA followed by Dunnett’s test).
Biochemical effects
Effect on brain antioxidant enzyme levels
The levels of the defensive antioxidant enzymes SOD and catalase were decreased in rats chronically treated with haloperidol. MAE administration (100– 300 mg/kg) normalized the haloperidol-induced decrease in brain SOD. MAE at doses of 200 and 300 mg/kg significantly (p < 0.05, p < 0.01) increased catalase compared with rats treated with haloperidol only ().
Table 1. Effects of coadministration of Morus alba leaf extract (MAE) on haloperidol-induced alterations in rat brain CAT, SOD, LPO, NO, and protein.
Lipid peroxidation assay
Chronic haloperidol treatment significantly (p < 0.05) increased lipid peroxidation, as indicated by a raise in brain MDA levels compared to the vehicle-treated group. Administration of MAE (100– 300 mg/kg) significantly (p < 0.05, p < 0.01) attenuated MDA levels as compared with rats treated with haloperidol only ().
Nitrite estimation
Oxidative stress increased the nitric oxide level, as indicated by a rise in the whole-brain nitrite level. Coadministration of MAE (100– 300 mg/kg) significantly (p < 0.05) reversed the nitrite activity as compared with rats treated with haloperidol only ().
Protein estimation
Oxidative stress causes protein damage leading to a decrease in total protein content in the brain. Administration of MAE (100– 300 mg/kg) normalized the protein content as compared with rats treated with haloperidol only ().
Discussion
The results of the present study indicate that MAE has a protective role against haloperidol-induced orofacial dyskinesia and oxidative stress.
Tardive dyskinesia (TD) is one of the major side effects of long-term neuroleptic treatment. The pathophysiology of this disability is still obscure. Despite decades of research, there is no uniformly safe and effective treatment (CitationGalili-Mosberg et al., 2000). Oxidative stress has been proposed as a pathogenic mechanism in TD. Neuroleptics, by blocking dopamine receptors, induce an increase in the turnover of dopamine, which may lead to the formation of reactive oxygen species (ROS) such as hydrogen peroxide and superoxide radicals (CitationNaidu et al., 2002). Increased levels of ROS might negatively affect both neurotransmission and cell viability that induce oxidative changes to lipids, resulting in an alteration in membrane function, protein damage, and reduction in intracellular antioxidant defense enzymes. Support for the “free radical hypothesis” comes from in vitro studies where haloperidol induced oxidative stress and vitamine E attenuated neuroleptic-induced changes in striatal monoamine metabolism and protected against neuroleptic-induced cell death (CitationAndreassen & Jorgensen, 2000).
In the present study, chronic treatment with haloperidol induced an increase in VCMs and tongue protrusions. The concomitant administration of MAE (100– 300 mg/kg) significantly attenuated the development of VCMs and tongue protrusions induced by long-term treatment with haloperidol. Rats treated with MAE alone did not show any significant behavioral changes, compared with the vehicle-treated group.
Of particular importance for the free radical hypothesis of TD, the present study demonstrates that chronic haloperidol administration causes a significant increase in levels of lipid peroxidation products and nitrite and decreased levels of natural cellular antioxidant enzymes such as SOD, which scavenges the superoxide ion by speeding up its dismutation, and CAT, which removes hydrogen peroxide, indicating that free radicals are effectively involved in the development of TD in rats.
The administration of haloperidol induced a marked decrease in brain SOD and CAT concentration. Concomitant treatment with MAE tended to normalize the haloperidol-induced decrease of SOD and CAT. Products of lipid peroxidation are also implicated in the pathophysiology of TD. ROS can initiate lipid peroxidation and DNA damage leading to neuronal loss if the antioxidant system is impaired (CitationBurger et al., 2003). Animals treated with haloperidol showed significantly increased levels of lipid peroxidation products, expressed as malondialdehyde (MDA), demonstrating the role of free radicals in TD. MAE significantly reduced the increased level of lipid peroxidation products in haloperidol-treated animals, suggesting a possible antioxidant action of MAE.
Nitric oxide (NO) is a multifunctional messenger. Nitrite is the stable end product of nitric oxide (NO) in a living system. Oxidative damage is indicated by raised nitrite activity in the brain (CitationGoyal & Kumar, 2007). Coadministration of MAE significantly reversed the nitrite activity as compared with haloperidol. The protein content of the brain was also normalized by MAE.
These results suggest that MAE may have free radical scavenging activity, thereby decreasing the lipid peroxidation levels and also sparing the antioxidant enzymes SOD and catalase.
The early hypothesis of TD was that it results from neuroleptic-induced alterations in γ-aminobutyric acid (GABA) transmission within the basal ganglia, and damage to striatal GABA-containing neurons. This is based on reports that rats with neuroleptic-induced oral movements had a decreased number of striatal GABAergic neurons. The GABA hypothesis is further supported by studies in rats where GABA agonists were shown to inhibit the development of neuroleptic-induced vacuous chewing movements. This hypothesis also provides a possible explanation as to why orofacial musculature is particularly affected in TD, by proposing that the striatal subregion controlling the oral system is especially vulnerable (CitationAndreassen & Jorgensen, 2000; CitationGalili-Mosberg et al., 2000). In our previous study, we showed a central nervous system (CNS)-depressant property of Morus alba. The behavioral experiments have lent support to the anxiolytic and muscle relaxant activities of Morus alba, which are possibly mediated through GABAergic transmission (CitationYadav et al., 2008). Therefore, the beneficial effect of MAE might be due to its GABAergic effects.
The findings of the present study demonstrate that concomitant MAE treatment is able to attenuate haloperidol-induced VCMs and tongue protrusions. MAE also mitigates haloperidol-induced biochemical alterations by tending to normalize SOD and catalase activity and decrease lipid peroxidation and nitrite. Thus, the study confirms that Morus alba has antioxidant activity. These results strengthen the oxidative stress hypothesis of TD and suggest a beneficial use of Morus alba in preventing this motor disorder.
Declaration of interest: The authors report no conflicts of interest.
References
- Andreassen OA, Jorgensen HA (1994): GM1 ganglioside attenuates the development of vacuous chewing movements induced by long-term haloperidol treatment in rats. Psychopharmacology 116: 517–522.
- Andreassen OA, Jorgensen HA (2000): Neurotoxicity associated with neuroleptic-induced oral dyskinesias in rats; implications for tardive dyskinesia. Prog Neurobiol 61: 525–541.
- Anonymous (1956): The Wealth of India. A Dictionary of Indian Raw Materials and Industrial Products. New Delhi, CSIR, pp. 429–437.
- Bishnoi M, Chopra K, Kulkarni SK (2008): Age-related susceptibility to chronic haloperidol-induced orofacial dyskinesia: Biochemical and neurochemical evidence. Indian J Pharmacol 39: 269–275.
- Burger ME, Alves A, Callegari L, Athayde FR, Nogueira CW, Zeni G, Rocha BT (2003): Ebselen attenuates reserpine-induced orofacial dyskinesia and oxidative stress in rat striatum. Prog Neuropsychopharmacol Biol Psychiatry 27: 135–140.
- Byambaa E, Kuninoro S, Takuya K, Keiko K, Yosuke Y (2005): Mulberry (Morus alba L) leaves and their major flavonol 3-(6-malonylglucoside) attenuate atherosclerotic lesion development in LDL receptor deficient mice. J Nutr 135: 729–734.
- Cheyl L (2006): Ethnomedicines used in Trinidad and Tobago for urinary problem and diabetes mellitus. J Ethnobiol Ethnomed 13: 45–51.
- Du J, He Z, Jiang R, Ye W, Xu H, But P (2003): Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry 62: 135–138.
- El-Beshbishy HA, Singab ANB, Sinkkonen J, Pihlaja K (2006): Hypolipidemic and antioxidant effects of Morus alba L. (Egyptain Mulberry) root bark fractions supplementation in cholesterol-fed rats. Life Sci 78: 2724–2730.
- Fukai T, Hano Y, Hirakura K, Nomura T, Uzawa J, Fukushima K (1985): Structures of two natural hypotensive Diels-Alder type adducts, mulberrofurans F and G, from the cultivated Mulberry tree. Chem Pharm Bull 33: 3195–3204.
- Fukai T, Satoh K, Nomura J, Sakagami H (2003): Antinephritis and radical scavenging activities of preflavonoids. Fitoterapia 74: 720–724.
- Galili-Mosberg R, Gil-Ad I, Weizman A, Melamed E, Offen D (2000): Haloperidol-induced neurotoxicity – possible implications for tardive dyskinesia. J Neural Transm 107: 479–490.
- Goyal R, Kumar A (2007): Protective effect of alprazolam in acute immobilization stress-induced certain behavioral and biochemical alterations in mice. Pharmacol Rep 59: 294–300.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannebaum SR (1982): Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Ann Biochem 126: 131–138.
- Hyuncheol OH, Eun-Kyung KO, Jung-Yang J, Myung-Hoon OH, Sung UK, Ki-Hong Kang, Ho-Sub Lee, Youn-Chul Kim (2002): Hepatoprotective and radical scavenging activities of preflavonoids, coumarin and stilbene from Morus alba. Planta Med 68: 932–940.
- Ikeda H, Adachi K, Hasegawa M, Sato M, Hirose N, Koshikawa N, Cools AR (1999): Effects of chronic haloperidol and clozapine on vacuous chewing and dopamine-mediated jaw movements in rats: evaluation of a revised animal model of tardive dyskinesia. J Neural Transm 106: 1205–1216.
- Kim SY, Gao JJ, Lee WC, Ryu KS, Lee KR, Kim YC (1999): Antioxidative flavonoids from the leaves of Morus alba. Arch Pharm Res 22: 81–88.
- Kokate CK (1994): Practical Pharmacognosy. Delhi, India, Vallabh Prakashan, pp. 104–111.
- Kono Y (1978): Generation of superoxide radical during autooxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186: 189–195.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951): Protein measurement with Folin–phenol reagent. J Biol Chem 193: 265–275.
- Luck H (1971): Catalase. In: Bergmeyer HU. Methods of Enzymatic Analysis. New York, Academic Press, pp. 885–893.
- Nadkarni AK (1976): Indian Materia Medica. Mumbai, India, Popular Prakashan, pp. 1292–1294.
- Naidu PS, Singh A, Kulkarni SK (2002): Carvedilol attenuates neuroleptic-induced orofacial dyskinesia: Possible antioxidant mechanisms. Br J Pharmacol 136: 193–200.
- Naidu PS, Singh A, Kulkarni SK (2003): Effect of Withania somnifera root extract on haloperidol-induced orofacial dyskinesia: possible mechanisms of action. J Med Food 6: 107–114.
- Singab ANB, El-beshbishy HA, Yonekawa M, Nomura T, Fukai T (2005): Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J Ethnopharmacol 100: 333–338.
- Tong Ho Kong, Hye Rim Oh, Sun Moon Jung, Jong Hoon Ryu, Mee Won Park, Yong Kon Park, Sun Yeou Kim (2006): Enhancement of neuroprotection of mulberry leaves (Morus alba L.) prepared by the anaerobic treatment against ischemic damage. Biol Pharm Bull 29: 270–274.
- Trease GD, Evans WC (1997): Pharmacognosy. New York, Harcourt, Brace & Company, p. 275, 343, 571.
- Wills ED (1966): Mechanism of lipid peroxide formation in animal tissues. Biochem J 99: 667–676.
- Wolfarth S, Ossowska K (1989): Can the supersensitivity of rodents to dopamine be regarded as a model of tardive dyskinesia? Prog Neuropsychopharmacol Biol Psychiatry 13: 799–840.
- Yadav AV, Kawale LA, Nade VS (2008): Effect of Morus alba L. (mulberry) leaves on anxiety in mice. Indian J Pharmacol 40: 32–36.
