Abstract
Two triterpenes, β-amyrin and 12-oleanene 3β, 21β-diol, were isolated as a mixture from the chloroform soluble fraction of an ethanol extract of Duranta repens Linn (Verbenaceae) stem. The structures of the two compounds were confirmed by analysis of their IR, 1H-NMR, 13C-NMR and LC-MS spectral data. The mixture of β-amyrin and 12-oleanene 3β, 21β-diol (compound 1) was highly effective against the larvae of the mosquito, Culex quinquefasciatus Say (Diptera: Culicidae), as a mosquitocide.
Introduction
Culex quinquefasciatus Say (Diptera: Culicidae) is a potential vector of Wuchereria bancrofti (Filarioidae), Otto Wucherer and Joseph Bancroft, the causative agent of human lymphatic filariasis (HLF) all over the globe, including Bangladesh (CitationBirley, 1993; CitationAhmed, 1994; CitationPailey et al., 1995). Mosquito-transmitted malaria, filariasis, yellow fever, dengue fever and Japanese B-encephalitis have great impact on public health in many countries in south and southeast Asian countries including Bangladesh (CitationBang, 1985; CitationShope, 1997). CitationHill (1997) estimated the number of people attacked by mosquito-borne diseases per year to be 100 million with mortality in more than a million cases. So, in order to prevent the transmission of mosquito-borne diseases, it is necessary to control mosquitoes using traditional insecticides, but in recent years it has been found that many mosquito species have already developed resistance to a number of chemical insecticides. In Bangladesh, C. quinquefasciatus is completely resistant to diazinon, fenitrothion, malathion, and primiphos-methyl and DDT (CitationGeorghiou & Lagunes-Tejeda, 1991; CitationWHO, 1992). An on going search for alternative pest control as well as vector control strategies have been developed by several researchers (CitationPimental et al., 1992; CitationHeckman, 1993; CitationCoats, 1994; CitationMulrennan, 1995; CitationSugiyama et al., 1996; CitationPeng et al., 1998).
Plant products or plant-derived compounds were reported as promising alternatives to synthetic insecticides in controlling insect pests (CitationRahuman et al., 2000). Vector control experts evaluated 344 plant species for their insecticidal, repellent, growth inhibiting, ovicidal activities and concluded with the suggestion that the plant products would be advantageous for field use in mosquito larvae control programs (CitationSukumar et al., 1991). In order to search for plant-derived compounds to be effective against mosquito larvae, a number of compounds have been reported including (5E)-ocimenone from Tagetes minuta Linn (Compositae) (CitationMaradufu et al., 1978), rotenone from Derris elliptica Benth (Fabaceae) (CitationAmeen et al., 1983), azadirachtin from Azadirachta indica A. Juss (Meliaceae) (CitationSchmutterer & Ascher, 1987), capillin from Artemisia nilagirica Linn (Asteraceae) (CitationBanerji et al., 1990), quassin from Quassia amara Linn (Simaroubaceae) (CitationEvans & Kaleysa Raj, 1991), neolignans from Piper decurrens Linn (Piperaceae) (CitationChauret et al., 1996) and goniothalamin from Bryonopsis laciniosa Linn (Cucurbitaceae) (CitationKabir et al., 2003).
CitationEl-Naggar and Mosallam (1987) reported that the extracts from Duranta repens Linn (Verbenaceae) had antifeedant and insecticidal properties not only against the larvae of Culex pipiens Linn and Spodoptera littoralis Boised (Lepidoptera: noctuidae) but also against the adults of Musca domestica Linn (Muscidae) and C. pipiens, respectively. CitationCastro and co-workers (1996) also reported that the fruits of D. repens were evaluated as antimalarials by oral and subcutaneous administration to mice infected with Plasmodium berghei Anka (Plasmodiidae). The chloroform extract of the fruits of the D. repens were reported to exhibit antifeedant activity against Heliotis armigera Hubner (Reoviridae), a polyphagus pest (CitationPatil et al., 2002). The aerial part of D. repens showed antiviral activity against Hepatitis A virus (CitationLobna et al. 2007). But information on the larvicidal activity of isolated compounds or extract of D. repens against C. quinquefasciatus is not available to date which has encouraged us to perform the present study including the isolation and structure elucidation of the bioactive compounds from this plant.
Materials and methods
Test insects
C. quinquefasciatus larvae were reared at 27° ± 1°C, 40–60% relative humidity and a 12:12 h light:dark photoperiod. Single egg rafts were placed in a number of 600-mL glass beakers (Duran®, Mainz, Germany) containing approximately 450 mL distilled water. The larvae were fed powdered Brewer’s yeast (Red Star®, Milwaukee, Wisconsin (WI)) at 10, 20, 40 and 80 mg per beaker everyday for first, second, third and fourth instars (different stages of a insect life), respectively. Water was changed everyday to avoid scum formation, which might create toxicity.
Test plant
Stems of D. repens were collected in June 2003 from the adjoining areas of Rajshahi University Campus, Bangladesh. The plant was identified by A.T.M. Nadiruzzaman, Department of Botany, University of Rajshahi, Bangladesh, where a voucher specimen (No. Alam 78, collection date 19.09.1997) has been deposited.
Extraction and isolation
The sun-dried and ground plant materials (1 kg) were macerated with ethanol (5 L). The concentrated ethanol extract was fractionated with diethyl ether and chloroform. The solvents were evaporated by rotary evaporator at 40°C and under reduced pressure to obtain ethanol (90 g), diethyl ether (20.8 g) and chloroform (15.6 g) extract as semisolid mass. The chloroform soluble fraction (5 g) was subjected to a column chromatography over silica gel eluting with n-hexane and ethyl acetate of increasing polarity, which gave a total of 33 fractions. On the basis of TLC (Thin Layer Chromatography) profile, the fractions were combined together. Compound 1 (480 mg) was obtained as amorphous powder from the fractions 4–15 eluted with n-hexane-ethyl acetate (2:1) by preparative TLC (mobile phase; n-hexane: ethyl acetate = 5:1) as visualized as pink spot when sprayed 1% vanillin in concentrated H2SO4.
Bioassay
The larvicidal effect of compound 1 was determined according to the protocol described by the CitationWHO (1975). The stock solution was prepared dissolving 10 mg of compound 1 in 1 mL of dimethylsulphoxide (DMSO). Then twenty-five laboratory reared first, second, third and fourth instars larvae were released into 100 mL glass beakers separately, containing 50 mL distilled water to which 50, 100, 200 and 400 μL stock solutions were added using capillary micro-pipettes (Wiretrol® II, Drummond Scientific Company, BROOMALL, Pennsylvania (PA)) to get the desired test concentrations (w/v), i.e. 10, 20, 40 and 80 ppm. Each concentration has three replications and three types of control were maintained: distilled water; distilled water + food medium and distilled water + solvent (DMSO), respectively. Each replication used 25 larvae. Control was raised similarly. The experiment was performed at 27° ± 1°C and 40-60% relative humidity. Brewer’s yeast was supplied as a larval food during the test periods for larval feeding. The cumulative mortality data were determined according to CitationAbbott’s (1925) formula and subjected to Probit analysis (CitationBusvine, 1971) for LC50 values.
Results and discussion
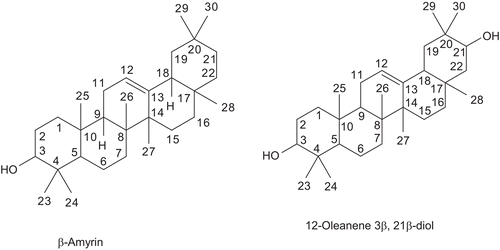
Compound 1 isolated from the chloroform fraction of ethanol extract of the stem of D. repens as a white amorphous powder, decomposed between 121°-125°C. IR spectrum showed O-H stretching band between 3445~3888 cm−1 and C-O- stretching vibration at 1099 cm−1. The C-H and >C=C-H stretching vibrations observed between 2877~2924 cm−1 and >C=C< stretching showed a strong band at 1689 cm−1. Although the TLC examination of compound 1 showed a single spot, but LC-MS and the NMR data (both 1H and 13C) suggested that it was not a single one. The 1H-NMR spectrum (500 MHz, CDCl3) of compound 1 showed two triplets (J = 3.6 Hz) at δH 5.26 and 5.30 that suggested the presence of two oleanane-type triterpenes having double bond at C12–C13. Comparison of 1H and 13C-NMR data with the published data and from LCMS, it was confirmed that the compound 1 is a mixture of two triterpenes, β-amyrin (major) and 12-oleanene 3β, 21β-diol (minor) in the ratio of 3:1 determined from the peak intensity.
Characterization of β-amyrin
The LC-APCI-MS showed a broad peak which gave two molecular ion [M+H]+ at m/z 427.3 and at m/z 443.1, corresponding to the molecular formula C30H50O and C30H50O2 respectively. The 1H-NMR spectrum showed the presence of an olefinic proton (H-12) at δH 5.30 and eight methyl group protons between 0.79-0.94 and a multiplet at δH 3.23 for an oxygenated proton (H-3). The H-12 (δH 5.30) coupled with the protons at δH 1.89 in the COSY experiment and was assigned as H-11 protons. In the HSQC experiment, H-12 showed direct correlation with a carbon at δC 124.2 (C-12). The 13C-NMR data of an authentic β-amyrin (CitationNdom et al., 2001) were compared to those of this part of compound 1 and found almost identical. Thus this part of compound 1 was identified as a major compound, β-amyrin, a common triterpene of plant origin. Though the values of H-12 and C-12 were slightly higher than those of authentic sample, which might be due to its existence as a component in a mixture ( and ).
Table 1. 1H-NMR (500 MHz, CDCl3) and 13C-NMR (125 MHz, CDCl3 ) data of compound 1.
Characterization of 12-oleanene 3β, 21 β-diol
The NMR spectrum of the minor compound showed many similarities to those of 12-oleanene- 3β, 22 β-diol (CitationRahman, 2002). The H-12 of the diol appeared as a triplet due to the coupling with H2-11 at δH 5.26 (J = 3.6 Hz) and H-3 at δH 3.27 (m). The proton at δH 0.74 (H-5) showed direct correlation to a methine carbon (C-5) at δC 54.2 in the heteronuclear single quantum coherence (HSQC) experiment and 3J correlation to methyl carbon at δC 17.1. The latter methyl carbon was also correlated to H-3 by 3J. The eight-methyl group signals were appeared between δH 0.80-1.15. The carbon chemical shifts of C-3 and C-12 were found to be 80.5 and 127.4, respectively, as evident from the HSQC experiment. The C-12 value of this minor component was slightly higher which was assumed to be due to its existence in a mixture.
The J modulated 13C-NMR data were very close to those of 12-oleanene-3β, 22β-diol (CitationRahman, 2002) except C-21 and C-22. While C-22 was oxygenated methine (δC 76.9) in case of 12-oleanene-3β, 22β-diol, one methine carbon was appeared at δC 80.2 in compound 1. As this chemical shift was very close to C-3 (δC 80.5) and two methyls existed at C-4, it was assumed that the carbon chemical shift at δC 80.2 must be C-21 while two methyls existed at C-20 instead of C-4. On this basis, this minor component was identified as 12-oleanene-3β, 21β-diol and the 1H-NMR and 13C-NMR data are presented in and . CitationMokboul et al. (1981) reported α-amyrin was isolated from D. repens. But both the compound β-amyrin and 12-oleanene 3β, 21β-diol are isolated for the first time from this plant and the minor one, 12-oleanene 3β, 21β-diol appeared to be a new compound.
Statistical data obtained from toxicity bioassays are presented in , which clearly showed that compound 1 (mixture of β-amyrin and 12-oleanene 3β, 21β-diol) was a potent larvicide against Culex quinquefqsciatus. With the increase of exposure time, the LC50 values of compound 1 decreased in all the instars tested. The increase in mortality with the increase of exposure period could be due to several factors, which may be acting separately or jointly. For example, the uptake of the active moiety of the compound could be time dependent, leading to a progressive increase in the titre of the plant-derived compounds tested and its effect on the larval body, the active moiety of the compound could get converted into more toxic metabolites in the larval integument and alimentary canal, resulting in time-dependent effects.
Table 2. Larvicidal action of compound 1 from D. repens on C. quinquefasciatus at different instars.
Antibacterial, antifungal, MIC, brine shrimp lethality, acute toxicity test on rats and insecticidal activity on Tribolium Castaneum (Herbst) was performed using compound 1 and showed promising antibacterial, antifungal, toxic and insecticidal activities such as MIC 32 μg/ml against Klebsiella sp.; 13 μg/disc zone of inhibition against Aspergillus flavus; LC50 1.21 ppm against brine shrimp larvae and LC50 90.6 μg/cm2 against 1st instars larvae of T. castaneum (Herbst) (CitationNikkon et al., 2008). The present results also seem to be very effective against C. quinquefasciatus. However, more comprehensive experiments are solicited in the future and if it is possible to produce their active analogues synthetically, which might prove to be more effective and more economical.
Acknowledgements
The authors are grateful to Professor Sohorab Ali, Department of Zoology, Rajshahi University, for helping Farjana Nikkon to perform the larvicidal activities and Professor Peter G Waterman, Centre for Phytochemistry and Pharmacology, Southern Cross University, Lismore, Australia, for running LC-MS and NMR spectra.
Declaration of interest
This work was supported by a Grant for Scientific Research (Fund No. BPROM/SHA-9/B-ANU-PRO/2000/324) from the Ministry of Science and Technology, Bangladesh.
References
- Abbott WS (1925): A method of computing the effectiveness of an insecticide. J Econ Entomol 18: 265–267.
- Ahmed SS (1994): Human filariasis in South and South-East Asia: A brief appraisal of our present knowledge. Proc Pakistan Congr Zool 14: 1–23.
- Ameen M, Shahjahan RM, Khan HR, Chowdhury AKA (1983): Toxicity of rotenone extracted from indigenous Derris roots on mosquito larvae. J Bangladesh Acad Sci 7: 39–47.
- Banerji A, Luthria DL, Kokate SD (1990): Toxicity of capillin, the insecticidal principle of Artemisia nilagirica Clarke. Indian J Exp Biol 28: 588–589.
- Bang YH (1985): Integrated management of urban mosquito vectors of human diseases. J Commun Dis 17: 8–10.
- Birley MH (1993): A historical review of malaria, kala-azar and filariasis in Bangladesh in relation to the flood action plan. Ann Trop Med Parasitol 87: 319–334.
- Busvine JR (1971): A Critical Review of the Techniques for Testing Insecticides, London, CAB International, p. 395.
- Castro O, Barrios M, Chinchilla M, Guerrero O (1996): Chemical and biological evaluation of the effect of plant extracts against Plasmodium berghei. Rev Biol Trop 44: 361–367.
- Chauret DC, Bernard CB, Arnason JT, Durst T, Krishnamurty HG, Sanchez-Vindas P, Moreno N, San-Roman L, Poveda L (1996): Insecticidal neolignans from Piper decurrens. J Nat Prod 59: 152–155.
- Coats JR (1994): Risks from natural versus synthetic insecticides. Ann Rev Ent 39: 489–515.
- El-Naggar MEA, Mosallam SS (1987): Insecticidal properties of some isolates from Duranta repens L. J Egyptian Soc Parasitology 17: 243–249.
- Evans DA, Kaleysa RR (1991): Larvicidal efficacy of quassin against Culex quinquefasciatus. Indian J Med Res 93: 324–327.
- Georghiou GP, Lagunes-Tejeda A (1991): The Occurrence of Resistance to Pesticides in Arthropods, Rome, FAO, p. 318.
- Heckman CW (1993): The fate of pesticides in heavily polluted aquatic system, in: Tilzer MM, Khondker M, eds, Hypertrophic and Polluted Freshwater Ecosystems: Ecological Bases for Water Resource Management. Proceedings of the International Symposium Limonol, 25–28 November, 1991, Department of Botany, University of Dhaka, Dhaka, Bangladesh, pp. 39–90.
- Hill DS (1997): The Economic Importance of Insects. London, Chapman and Hall, p. 395.
- Kabir KE, Khan AR, Mosaddik MA (2003): Goniothalamin – a potent mosquito larvicide from Bryonopsis laciniosa L. J Appl Ent 127: 112–115.
- Lobna MAS, Naglaa MN, Abdellaaty AS (2007): Phytochemical investigation and antiviral activity of Duranta repens. J Appl Sci Res 3: 1426–1433.
- Makboul AM, Abdul-Baki AM (1981): Flavonoids from the leaves of Duranta plumieri. Fitoterapia 52: 219–220.
- Maradufu A, Lubega R, Dorn F (1978): Isolation of (5E)-ocimenone, a mosquito larvicide from Tagetes minuta. Lloydia 41: 181–183.
- Mulrennan JJA (1995) Vector control without chemical: A public health perspective. J Am Mosq Contr Assoc 11: 256–257.
- Ndom JC, Vardamides KJC, Wansi JD, Kamdem AW, Mobafor JT, Fomum ZT (2001): Constituents of Erythrina sigmoidea. Bull Chem Soc Ethiop 15: 151–156.
- Nikkon F, Habib MR, Karim MR, Hossain MS, Mosaddik MA, Haque ME (2008): Antishigellosis and cytotoxic potency of crude extracts and isolated constituents from Duranta repens. Mycobiology 36: 173–177.
- Pailey KP, Hoti SL, Manonmani AM, Balaraman K (1995): Longevity and migration of Wuchereria bancrofti infective larvae and their distribution pattern in relation to the resting and feeding behaviour of the vector mosquito, Culex quinquefasciatus. Ann Trop Med Parasitol 89: 39–47.
- Patil VJ, Deshmukh MB, Maner MI (2002): Antimicrobial and antifeedant activity of the extract of the plant Duranta repens. J Biotech Agric Inds Enviro 5: 65–67.
- Peng Y, Song J, Tian G, Xue Q, Ge F, Yang J, Shi Q (1998): Field evaluations of Romanomermis yunanensis (Nematoda: Mermithidae) for control of Culicinae mosquitoes in China. Fundam Appl Nematol 21: 227–232.
- Pimental D, Acquay H, Biltonen M, Rice P, Silva M, Nelson J, Lipner V, Giordano S, Horowitz A, D’Amoer M (1992): Environmental and economic costs of pesticide use. Bioscience 42: 750–760.
- Rahman MM (2002): Phytochemical and antimicrobial studies on some species of Bangladeshi Leguminaceae and Rutaceae, Ph.D. Thesis, University of Strathclyde, pp. 225–228.
- Rahuman AA, Gopalakrishnan G, Salim GB, Arumugam S, Himalayan B (2000): Effect of Feronia limonia on mosquito larvae. Fitoterapia 71: 553–555.
- Schmutterer H, Ascher KRS (1987): Natural pesticides from the neem tree and other tropical plants, in: Proceedings of the Third International Neem Conference, Nairobi, 10–15 July 1986. Eschborn, GTZ, p. 703.
- Shope RE (1997): Concepts of control of Japanese encephalitis and dengue. Southeast Asian J Trop Med Pub Health 28: 131–134.
- Sugiyama A, Takagi M, Maruyama K (1996): A laboratory experiment of the predation by possible predators on Culex tritaeniorhynchus larvae. Trop Med 38: 7–12.
- Sukumar K, Perich MJ, Boobar LR (1991): Botanical derivatives in mosquito conrtol, a review. J Am Mosq Contr Assoc 7: 210–237.
- WHO (World Health Organization) (1975): Instruction for determining the susceptibility or resistance of mosquito larvae to insecticides. Mimeographed document. WHO/VBC/1975, p. 583.
- WHO (1992): Vector resistance to pesticides, Fifteenth Report of the WHO Expert Committee on Vector Biology and Control. WHO Tech Rep Ser 818: 1–62.