Abstract
Aristolochia baetica L. (Aristolochiaceae) and Origanum compactum Benth. (Lamiaceae) are native plants of Morocco used in traditional medicine. In order to systematically evaluate their potential activity on human breast cancer, four different polarity extracts from each plant were assessed in vitro for their antiproliferative effect on MCF-7 cells. As a result, several extracts of those plants showed potent cell proliferation inhibition on MCF-7 cells. Chloroform extract of A. baetica (IC50: 216.06 ± 15 μg/mL) and ethyl acetate of O. compactum (IC50: 279.51 ± 16 μg/mL) were the most active. Thin layer chromatography examination of the bioactive extracts of A. baetica and O. compactum showed the presence of aristolochic acid and betulinic acid, respectively. These results call for further studies of these extracts.
Introduction
Cancer is a worldwide problem which is a major concern for public health. Breast cancer is ranked first of cancers in women (affecting 1 in 10 women) with more than 540,000 new cases each year in the world (CitationGhazali et al., 2000). It is a frequent and serious pathology since a quarter of million women die because of it each year (CitationGhazali et al., 2000). Consequently, there is a significant need to improve current breast cancer therapies and to search for new therapies.
Phytotherapy has been practiced since antiquity in Africa, Asia, Europe, and the Americas (CitationWargovich et al., 2001). During the past decade, the use of herbs and related products has increased from 34% in 1990 to 42% in 1995, with related out-of-pocket costs of about $27 billion (CitationRichardson, 2001). Throughout the centuries, several treatments and remedies have been tested for antitumor potential. Plants have provided many effective anticancer agents in current use such as irinotecan, taxanes, topotecan, vinblastine, vincristine, etc. (CitationPezzuto, 1997; CitationKinghorn et al., 1999; CitationLee, 1999). Plant-derived products are excellent sources for the discovery and development of new anticancer agents (CitationAbdulaev, 1993).
In Moroccan traditional medicine, the use of herbs, in the form of decoctions or infusions, is a practice among the native population. Nowadays, the rural populations are still using herbs, and their use is increasing even in urban populations. Although there is an important local ethnobotanical bibliography (CitationHmamouchi, 1999; CitationMerzouki et al., 2000), few reports describe the use of medicinal plants to treat cancer.
Aristolochia baetica L. (Aristolochiaceae) and Origanum compactum Benth. (Lamiaceae) are well-known Moroccan plants with cancer-related ethnobotanical use. In this study we have explored four different polarity extracts from each of those plants on the human breast cancer cell line MCF-7, in order to confirm or discourage their popular use.
Materials and methods
Plant material
A. baetica and O. compactum were collected during March 2004 from their natural grounds in Morocco and taxonomically identified by M. Hmamouchi, National Institute of Medicinal and Aromatic Plants, Taounate. A voucher specimen (respectively FMP-27 and FMP-79) is deposited at the herbarium of the Faculty of Medicine and Pharmacy, Rabat, Morocco. The ethnobotanical information of the plants assayed is presented in (CitationHmamouchi, 1999; CitationHmamouchi et al., 2000; CitationMerzouki et al., 2000; CitationRuffa et al., 2002).
Table 1. Ethnobotanical data and antiproliferative effect of extracts from the studied plants.
Extraction procedure
A. baetica (roots) and O. compactum (aerial parts) were dried and crushed, and then 500 g of each plant were extracted in a Soxhlet for at least 48 h by four organic solvents according to an increasing gradient of polarity: hexane, chloroform, ethyl acetate, and methanol.
The recovered extracts were concentrated by the rota-vapor and were stored at -20°C until analysis.
Cell line, cell culture and treatment
Human breast tumor cell line (MCF-7) was kindly provided by G. Habrioux (Laboratoire de Biochimie, Faculté de Pharmacie de Limoges, France). MCF-7 cells were seeded at density of 6.104 cells/mL in 75 cm2 tissue culture flasks, grown in DMEM medium (Gibco, Cergy-Pontoise, France) supplemented with 10% fetal bovine serum (Gibco), 1% l-glutamine and 1% penicillin/streptomycin mixture (Gibco). Cultures were maintained in a humified atmosphere with 5% CO2 at 37°C to subconfluence. Fresh medium was supplied every 48 h. The doubling time of MCF-7 cells was estimated to be 18 h by growth curve studies.
Cells were allowed to grow for 24 h in culture medium prior to exposure to plant extracts for 24, 48, and 72 h. A stock solution of 50 mg/mL of extracts was prepared in DMSO (Sigma Aldrich, l’Isle d’Abeau Chesnes, France) and diluted in culture medium to give a final concentration 1 to 1000 μg/mL. The same amount of DMSO was added to control cells.
Cell proliferation assay
The medium was aspirated from MCF-7 cells grown to about 90% confluence. Cells were washed with PBS, trypsinized, counted with a hemocytometer and subcultured into 96-well plates with 6.103 cells per well in a 100 μL medium. After 24 h incubation at 37°C in a 5% CO2 incubator, the seeding medium was removed and replaced by plant extracts diluted in medium to a final concentration ranging from 1 to 1000 μg/ mL. Measurement of the cell proliferation was determined after 24, 48, and 72 h of the treatment using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MTT experiments were performed in six assays as previously described (CitationMoalic et al., 2000). Briefly, 10 μL of the MTT solution (5 mg/ mL in distilled water) were added on cultured cells. After 4 h of incubation at 37°C in wet atmosphere, in darkness, 100 μL of a lysis solution (SDS 10%; HCl 0.01N) were added into the wells, and the plate was then incubated at 37°C overnight. The absorbance was read at wavelength 550 nm using a microtiter plate reader (Multiskan EX, Labsystems, Cergy-Pontoise, France).
Negative and positive controls of the test correspond, respectively, to untreated MCF-7 cells and cells treated by paclitaxel (Cooper Maroc Laboratoires, Casablanca, Morocco); only exposed to vehicle (DMSO). The absorbance of the negative control wells was taken as 100 % and the results were expressed as a percentage of negative control proliferation: (mean absorption of plant extract treated wells/mean absorption of negative control wells) ± standard deviation.
Determination of IC50 concentration of plant extracts
The absorbance values obtained per treatment were converted to percentage proliferation. Regression analysis was performed on MTT assay proliferation data and the resultant equation was used to compute the inhibition concentration required to produce a 50% reduction in cell proliferation (IC50). The values of the IC50 obtained were confirmed by blue exclusion assay data.
Thin layer chromatography analysis
Preparation of standard solutions: aristolochic acid I (1 mg) and betulinic acid (1 mg) are dissolved in methanol (1 mL) to obtain standard solution respectively for thin layer chromatography (TLC) systems I and II; aristolochic acid I and betulinic acid for crude drug purity tests (Sigma) were used. All the other solvents were of analytical grade (Sigma).
TLC conditions: the TLC plates were RP-18F254S (Art. 15389, Merck, Darmstadt, Germany), and the solvent systems were I) acetonitrile/methanol/water mixture solution (3:1:1), and II) hexane/chloroform mixture solution (7:3). Volume of spot, 10 μL; developing distance, about 10 cm; method of detection, examine under UV light (wavelength: 254 nm) in the dark for system I; staining with iodine vapour and examining under UV light (wavelength: 365 nm) for system II.
Statistical analysis
The median and standard deviation (SD) were calculated using Excel (Microsoft Office, Version 98). Statistical analysis of differences was carried out by analysis of variance (ANOVA). A P- value of less than 0.05 was considered to indicate significance.
Results
Yields of extracts
The percentage yields of the A. baetica organic extracts were hexane (1.36%), chloroform (1.33%), ethyl acetate (0.65%), and methanol (24.4%). Moreover, the yields of the O. compactum organic extracts were hexane (4.7%), chloroform (5.68%), ethyl acetate (3.33%), and methanol (13.06%).
Antiproliferative activity of plant extracts
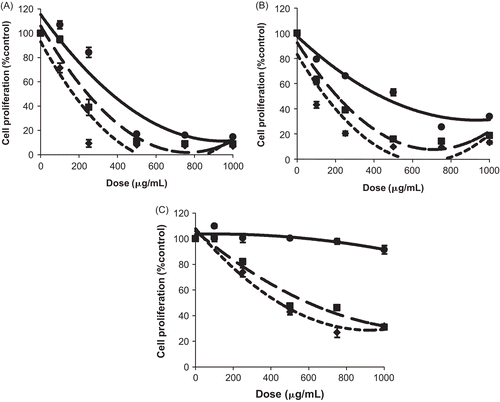
Results of the MTT test revealed for A. baetica, that except for the methanol extract (data not shown), all the three other extracts induced a dramatic decrease into MCF-7 cell proliferation, especially at times 48 and 72 h ().
Figure 1. Antiproliferative effect of Aristolochia baetica L extracts on MCF-7 cells. Cells were treated with various concentrations for 24 (—), 48 (-·-), and 72 h (…). (A) MCF-7 cells are treated by hexane extract. (B) MCF-7 cells are treated by chloroform extract. (C) MCF-7 cells are treated by ethyl acetate extract. Results are presented as a percentage of negative control (untreated cells) proliferation. Values were expressed as mean ± SD of six experiments (p-value relative to control group: p <0.05).
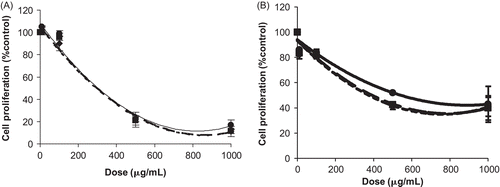
As far as the O. compactum is concerned, the methanol extract and ethyl acetate extract showed an antiproliferative effect on MCF-7 cells (), whereas the two other extracts were not exploitable (the hexane extract was not completely soluble in DMSO and the chloroform extract interfered with the wavelength used in the MTT test).
Figure 2. Antiproliferative effect of Origanum compactum Benth. extracts on MCF-7 cells. Cells were treated with various concentrations for 24 h (—), 48 h (-·-), and 72 h (…). (A) MCF-7 cells are treated by ethyl acetate extract. (B) MCF-7 cells are treated by methanol extract. Results are presented as percentage of negative control (untreated cells) proliferation. Values were expressed as mean ± SD of six experiments (p-value relative to control group: p <0.05).
The concentrations of plant extracts required to produce a 50% reduction in cell proliferation (IC50) were computed by a regression analysis using data from the MTT assays ().
For the three bioactive extracts of A. baetica, the IC50 values decrease according to time of treatment. The comparison of IC50 values of these three extracts showed that the chloroform extract was the most active at 48 and 72 h of treatment (IC50: 216 μg/mL and 146 μg/mL at 48 and 72 h), followed-up respectively by the hexane extract (IC50: 251 μg/mL at 48 h and 168 μg/mL at 72 h) and the ethyl acetate extract (IC50: 608 μg/mL and 435 μg/mL at 48 and 72 h).
For the two active extracts of O. compactum, there was an important decrease in IC50 values between 24 and 48 h of treatment. On the other hand, the value of 72 h was almost similar to that of 48 h. Thus, for this plant the extract of ethyl acetate was more active (IC50: 279 μg/mL at 48 h and 275 μg/mL at 72 h) than the methanol extract (IC50: 382 μg/mL and 374 μg/mL at 48 and 72 h).
The Trypan blue exclusion study confirmed the IC50 values obtained for the two studied plants.
Our results showed that the responsible compound for the maximum antiproliferative effect for A. baetica and O. compactum seemed to be concentrated respectively in the chloroform and ethyl acetate extracts.
Thin layer chromatography assay
For identification of aristolochic acid and betulinic acid respectively in the three bioactive extracts of A. baetica (hexane, chloroform, and ethyl acetate extracts) and in the two active extracts of O. compactum (methanol and ethyl acetate extracts); two standard solutions were prepared using aristolochic acid and betulinic acid as marker compounds. Two TLC systems were used.
For the A. Baetica extracts, to separate aristolochic acid I from crude drugs, the solvent system I acetonitrile/methanol/water mixture solution (3:1:1) was used. When each crude drug was separated using this system, a single spot was observed at the same Rf value as that of standard solution for the hexane, chloroform, and ethyl acetate extracts.
As far as the O. compactum extracts were concerned, when each crude drug was separated using the solvent system II hexane/chloroform mixture solution (7:3), a single spot was observed at the same Rf value as that of the betulinic acid marker for the two active extracts.
Consequently, TLC examination of the bioactive extracts of A. baetica and O. compactum showed the presence of aristolochic acid I and betulinic acid, respectively.
Discussion
This study revealed the antiproliferative capacity of A. baetica and O. compactum on MCF-7 breast cancer cell line.
This difference in results between the extracts from each of those plants is normal since every solvent extract differs, comprising a set of products of different chemical structure according to their polarity and affinity. The comparison of IC50 values seems to indicate that the responsible compound for this action for A. baetica and O. compactum was concentrated, respectively, in the chloroform and ethyl acetate extracts. The two plants may have some kind of antiproliferative activity. Nevertheless, A. baetica was the most active.
Although the procedure of extraction was the same, O. compactum exhibited this effect in the polar extracts, as opposed to A. baetica, which translates the difference of the responsible compounds for this activity between these two plants.
Indeed, it was reported that the Aristolochia spp. have an antiproliferative effect. Thus, it was described that A. triangularis inhibited strongly the proliferation of human epidermoid carcinoma (CitationMongelli et al., 2000), and A. macroura Gomez showed cytotoxic effect against a human hepatocellular carcinoma (CitationRuffa et al., 2002).
It has been described that aristolochic acid or related compounds contained in the Aristolochia spp. (CitationHu et al., 2004), have cytotoxic effect against numerous cancer cell lines (CitationViel & Dore, 1972; CitationWu et al., 1999, Citation2000; CitationGoun et al., 2002). Thin layer chromatography examination of the three active extracts of A. baetica indicated the presence of aristolochic acid I. Consequently, the responsible compound for the antiproliferative effect of this plant, shown in our study, seems to be aristolochic acid I.
As far as the Origanum spp. is concerned, a previous study reported the in vitro anti-hepatoma activity of an herb of the same botanical family, named O. marjorana L. with an IC50 equal to 1511 μg/mL (CitationLiang et al., 2002), which was very high compared to those of our study (IC50: 279 μg/mL). This can be explained especially by the screening of an aqueous extract (decoction) as opposed to our study in which we tested an organic extract.
The chemistry of the whole plant of O. compactum was not studied in as detailed a way as that of its essential oil. Oleanolic, ursolic and betulinic acids belong to pentacyclic triterpenes and have been found in this plant (CitationBellakhdar et al., 1988; CitationDe Spiridon, 2002). These terpenes may exist in the form of free acids or as aglycones for triterpenoid saponins. They have many important pharmacological effects (CitationWojciak-Kosior, 2007).
In the literature, there are numerous data on antitumor activity of betulinic acid, especially in melanoma cancer (CitationPisha et al., 1995), but also in colorectal cancer (CitationJung et al., 2007), prostate cancer (CitationChintharlapalli et al., 2007), ovarian cancer, and lung carcinoma (CitationZuco et al., 2002).
Our thin layer chromatography examination of the bioactive extracts of O. compactum showed the presence of the betulinic acid, which suggests that this compound is a contributor to the antiproliferative effect of O. compactum.
In conclusion, our study provides the first evaluation of those plants tested as chemopreventive interest, and may partly justify their cancer-related ethnobotanical use. Furthermore, isolation of the active principles is in progress to determine structures and mechanism of action.
Acknowledgements
The authors gratefully acknowledge Dr. M. Mimouni (Service des Laboratoires, Institut National d’Oncologie, Rabat, Maroc) for his helpful discussion and review of this article.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. This work was supported by EGIDE (Centre Français pour l’Accueil et les Echanges Internationaux).
References
- Abdulaev FI (1993): Plant-derived agents against cancer, in: Gupta SK, ed., Pharmacology and Therapeutics in the New Millennium, New Delhi, Narosa Publishing House, pp. 345–354.
- Bellakhdar J, Passannanti S, Paternostro MP, Piozzi F (1988): Constituents of Origanum compactum. Planta Med 54: 94–94.
- Chintharlapalli S, Papineni S, Ramaiah SK, Safe S (2007): Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res 67: 2816–2823.
- De Spiridon EK (2002). Oregano: The Genera Origanum and Lippia. London, Taylor and Francis, pp. 92–95.
- Ghazali M, Boumezgou K, Morsad F, Brams S, Abbassi H, Matar N, El Mansouri A (2000): Epidémiologie du cancer du sein(Epidemiology of breast cancer). Les Cahiers du Médecin 3: 17–22.
- Goun E, Cunningham G, Solodnikov S, Krasnykch O, Miles H (2002): Antithrombin activity of some constituents from Origanum vulgare. Fitoterapia 73: 692–694.
- Hmamouchi M (1999). Les Plantes Médicinales et Aromatiques Marocaines (The Moroccan Medicinal and Aromatic Plants), Morocco, Edition Fédala, pp. 53–132.
- Hmamouchi M, Lahlou M, Agoumi A (2000): Molluscicidal activity of some Moroccan medicinal plants. Fitoterpia 71: 308–314.
- Hu SL, Zhang HQ, Chan K, Mei QX (2004): Studies on the toxicity of Aristolochia manshuriensis (Guanmuton). Toxicology 198: 195–201.
- Jung GR, Kim KJ, Choi CH, Lee TB, Han SI, Han HK, Lim SC (2007): Effect of betulinic acid on anticancer drug-resistant colon cancer cells. Basic Clin Pharmacol Toxicol 101: 277–285.
- Kinghorn AD, Farnsworth NR, Doel Soejarto D, Cordell GA, Pezzuto JM, Udeani GO, Wani MC, Wall ME, Navarro HA, Kramer RA, Menendez AT, Fairchild CR, Lane KE, Forenza S, Vyas DM, Lam KS, Shu YZ (1999): Novel strategies for the discovery of plant-derived anticancer agents. Pure App Chem 71: 1611–1618.
- Lee KH (1999): Anticancer drug design based on plant-derived natural products. J Biomed Sci 6: 236–250.
- Liang TL, Li TL, Lien CC, Chun CL (2002): In vitro anti-hepatoma activity of fifteen natural medicines from Canada. Phytother Res 16: 440–444.
- Merzouki A, Ed-derfoufi F, Molero MJ (2000): Contribution to the knowledge of Rifian traditional medicine. II: Folk medicine in Ksar Lakbir district NW Morocco. Fitoterapia 71: 278–307.
- Moalic S, Liagre B, Labrousse F, Beneytout JL (2000): Enhanced apoptosis in retrovirally transfected osteosarcoma cells after exposure to sodium butyrate. Int J Oncol 16: 695–700.
- Mongelli E, Pampuro S, Coussio J, Salomon H, Ciccia G (2000): Cytotoxic and DNA interaction activities of extracts from medicinal plants used in Argentina. J Ethnopharmacol 71: 145–151.
- Pezzuto JM (1997): Plant-derived anticancer agents. Biochem Pharmacol 53: 121–133.
- Pisha E, Chai H, Lee I.-S, Chagwedera TE, Farnsworth N, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown DM, Wani MC, Wall ME, Hieken TJ, das Gupta TK, Pezzuto JM (1995): Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med 1: 1046–1051.
- Richardson MA (2001): Biopharmacologic and herbal therapies for cancer: Research update from NCCAM. J Nutr 131: 3037S–3040S.
- Ruffa MJ, Ferraro G, Wagner ML, Calcagno ML, Campos RH, Cavallaro L (2002): Cytotoxic effect of Argentine medicinal plant extracts on human hepatocellular carcinoma cell line. J Ethnopharmacol 79: 335–339.
- Viel C, Dore JC (1972): New synthetic cytotoxic and antitumoral agents derived from aristolochic acid. Farmaco 27: 257–312.
- Wargovich MJ, Woods C, Hollis DM, Zander ME (2001): Herbs, cancer prevention and health. J Nutr 131: 3034S-3036S.
- Wojciak-Kosior M (2007): Application of high performance thin-layer chromatography to separation of oleanolic, ursolic and betulinic acids. J Pre-Clin Clin Res 1: 176–178.
- Wu TS, Chan YY, Leu YL, Chen ZT (1999): Sesquiterpene esters of aristolochic acid from the root and stem of Aristolochia heterophylla. J Nat Prod 62: 415–418.
- Wu TS, Leu YL, Chan YY (2000): Constituents from the stem and root of Aristolochia kaempferi. Biol Pharm Bull 23: 1216–1219.
- Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti-Passerini C, Formelli F (2002): Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal -cells. Cancer Lett 175, 17–25.