Abstract
Citronellal is a monoterpene reported to be a major component of the essential oils in various aromatic species of plants. The present study evaluated the central nervous system depressant and antinociceptive properties of citronellal through behavioral experimental models. Following intraperitoneal injection, citronellal induced the reduction of spontaneous activity, ataxia, analgesia, and sedation. In pentobarbital-induced hypnosis, CTL (citronellal) at 50, 100, and 200 mg/kg (i.p.) significantly increased sleeping time (88.0 ± 11.4, 100.2 ± 16.4, and 119.5 ± 20.9 min) when compared to vehicle solution injections (43.0 ± 6.1). Citronellal (100 and 200 mg/kg, i.p.) significantly reduced the number of writhes (66.4 and 81.9%) in a writhing test and the number of paw licks during phase 1 (47.0 and 66.8%) and phase 2 (71.1 and 79.2%) of a formalin test when compared to control group animals. In addition, the results of a hot plate test showed central analgesic properties for citronellal (p < 0.05). These results indicate depressant, hypnotic, and antinociceptive properties of this monoterpene.
Introduction
Pain is a sensorial modality, which in many cases represents the only symptom for the diagnosis of several diseases, and often has a protective function. Throughout history man has used many different forms of therapy for the relief of pain, and medicinal herbs are highlighted due to their wide popular use. An example is Papaver somniferum L. (Papaveraceae), from which morphine has been isolated, and is regarded as the prototype of opiate analgesic drugs (CitationAlmeida et al., 2001). This fact has stimulated a considerable amount of research into a new drug for pain treatment, and consequently numerous herbal medicines have been recognized as active on the central nervous system (CNS) (CitationPhillipson, 2001). In addition, they have been described to have a hypothetical potential to affect chronic conditions such as anxiety, depression, headaches, pain, or epilepsy, which do not respond well to conventional treatments (CitationCarlini, 2003).
Monoterpenes are the main chemical constituents of the essential oils of these medicinal herbs, and are mixtures of odoriferous components obtained by steam distillation or solvent extraction from a large variety of aromatic plants. They are found in edible as well as medicinal plants with a therapeutic property (CitationCarneiro et al., 1997). Additionally, monoterpenes and their derivative compounds exhibit several types of pharmacological properties, such as anxiolytic (CitationSilva et al., 2007), antinociceptive (De Sousa et al., Citation2007b), sedative (CitationElisabetsky et al., 1995; De Sousa et al., Citation2006a, Citation2007a), antidepressant (De Sousa et al., Citation2006b), and anticonvulsant (CitationViana et al., 2000; De Sousa et al., Citation2006c, Citation2007c). In this regard, citronellal (3,7-dimethyl-6-octen-1-al) as a monoterpene is a major component of the essential oils in various aromatic species, such as Cymbopogon winterianus Jowitt (Java citronella) (CitationQuintans-Júnior et al., 2008), Corymbia citriodora (Hook.) K.D.Hill, and C. nardus L. (CitationLenardão et al., 2007). This monoterpene presents many applications in popular medicine and aromatherapy. Citronellal is used in the perfumery, cosmetic, and soap industries (CitationLawless, 2002). Along with citral, geranial, linalool, and citronellol, citronellal (CTL) is one of the most important terpenes.
The purpose of the present study was to evaluate the effect of citronellal on the central nervous system and its antinociceptive activity using acetic acid-induced writhing, formalin, and hot plate tests on mice.
Materials and methods
Chemicals
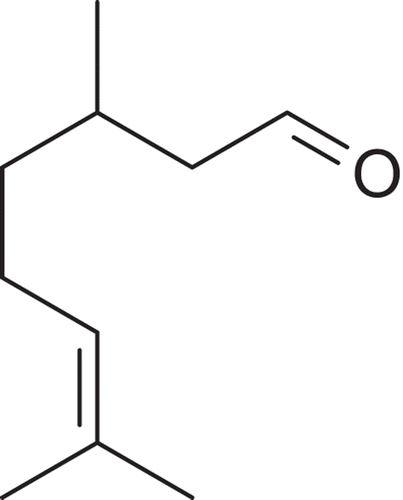
Pentobarbital, acetic acid, and polyoxyethylene-sorbitan monolate (Tween 80) were purchased from Sigma (USA). Morphine, naloxone, and acetylsalicylic acid were purchased from União Química (Brazil). (RS)-(±)-Citronellal (98% purity) () was purchased from Dierberger (Brazil).
Animals
Male Swiss mice (30–35 g), 2–3 months of age, were used throughout this study. The animals were randomly housed in appropriate cages at 25 ± 2°C on a 12 h light/dark cycle (lights on 06:00–18:00 h) with free access to food (Purina) and water. They were used in groups of 10 animals each. All experiments were carried out between 09:00 and 16:00 h in a quiet room. Experimental protocols and procedures were approved by the Animal Care and Use Committee (CEPA/UFS N° 12/08) at the Universidade Federal de Sergipe.
Behavioral effects
Behavioral screening of the mice was performed following parameters described by CitationAlmeida et al. (1999), and animals were observed at 0.5, 1.0, and 2.0 h after intraperitoneal (i.p.) administration of citronellal (50, 100, and 200 mg/kg, i.p.).
Pentobarbital-induced hypnosis
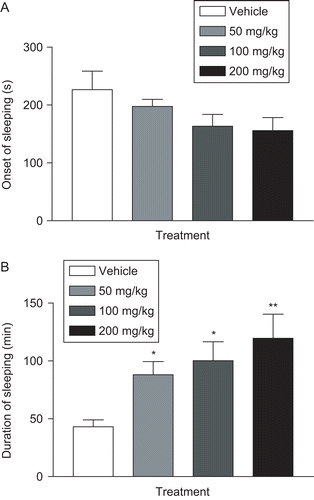
Sodium pentobarbital, at a hypnotic dose of 50 mg/ kg (i.p.), was injected into four groups (n = 10) of mice 30 min after pretreatment with saline/Tween 80 0.2% (vehicle) and CTL (50, 100, and 200 mg/kg, i.p.), respectively. The latency (interval between injection of sodium pentobarbital and loss of the righting reflex) and duration of sleeping time (interval between loss and recovery of the righting reflex) were recorded (CitationElisabetsky et al., 1995).
Antinociceptive activity
Acetic acid-induced writhing
This test was done using the method described by CitationKoster et al. (1959) and CitationBroadbear et al. (1994). Muscular contractions were induced by i.p. injection of a 0.85% solution of acetic acid (0.25 mL/animal) to a group of 10 mice. The number of muscular contractions was counted for 15 min after injection and the data represent the average of the total number of writhes observed. CTL was administered in doses of 50, 100, and 200 mg/kg (i.p.). The reference drug, acetylsalicylic acid (ASA), was solubilized in saline plus one drop of Tween 80 0.2% (vehicle) (200 mg/kg) and was administered intraperitoneally to different groups of mice 0.5 h before acetic acid injection.
Formalin test
The observation chamber was a glass box of 30 cm diameter on a transparent acrylic plate floor. Beneath the floor, a mirror was mounted at a 45° angle to allow clear observation of the paws of the animals. The animals were treated with vehicle (saline plus one drop of Tween 80 0.2%), CTL (50, 100, and 200 mg/kg, i.p.), or the reference drug (ASA 200 mg/kg, i.p.) 0.5 h before the formalin injection. Each mouse was placed in the chamber more than 5 min before treatment in order to allow acclimatization to the new environment. The formalin test was carried out as described by CitationHunskaar and Hole (1987). Twenty microliters of a 2.5% formalin solution (0.92% formaldehyde) in phosphate buffer (pH 7.2) were injected into the dorsal surface of the left hind paw using a microsyringe with a 26-gauge needle. Each animal was then returned to the chamber and the amount of time that the animal spent licking the injected paw was considered to be indicative of pain. Two distinct phases of intensive licking activity were identified: an early acute phase and a late or tonic phase (0–5 and 15–30 min after formalin injection, respectively).
Hot plate test
The hot plate test described by CitationJacob et al. (1974) and by CitationJacob and Ramabadran (1978) was used. The animals were placed on an aluminum plate that was adapted to a water bath at 55 ± 0.5°C. The reaction time was noted by observing the licking of the hind paws at basal, 0.5, 1.0, 1.5, and 2.0 h after i.p. administration of 50, 100, and 200 mg/kg of CTL or vehicle (saline plus one drop of Tween 80 0.2%) to different groups of 10 mice. Morphine, 5 mg/kg (i.p.), was used as the reference drug. The effect of pretreatment with naloxone (0.5 mg/kg, i.p.) on the antinociception produced by CTL (100 and 200 mg/kg) and morphine (5 mg/kg, i.p.) was determined.
Statistical analysis
The obtained data were evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s or Fisher’s tests. In all cases, differences were considered significant if p < 0.05. The percent of inhibition by an antinociceptive agent was determined for the acetic acid-induced writhing and formalin tests using the following formula (CitationReanmongkol et al., 1994):
Results
Behavioral effects
CTL at doses of 50, 100, and 200 mg/kg (i.p.) showed depressant activity on the CNS based on the following behavioral alterations in animals after 30, 60, and 120 min of elapsed time after treatment: decrease of spontaneous activity, palpebral ptosis, ataxia, analgesia, and sedation.
Pentobarbital-induced hypnosis
As shown in , CTL at doses of 100 and 200 mg/kg (i.p.) did not affect the latency of pentobarbital-induced hypnosis. CTL at 50, 100, and 200 mg/kg (i.p.) significantly (p < 0.05) increased the sleeping time compared to control group animals ().
Acetic acid-induced writhing
shows that CTL significantly (p < 0.01) reduced, in a dose-dependent manner, the number of writhing movements induced by the i.p. administration of acetic acid solution. Although these results are significant, the inhibition (%) of nociceptive behavior by the 50, 100 and 200 mg/kg (61.4, 66.4, and 81.9%) doses was inferior to that produced by the reference drug, aspirin 200 mg/kg (87.8%).
Table 1. Effect of citronellal (CTL) or aspirin on writhing induced by acetic acid.
Formalin test
In the first and second phases, CTL significantly reduced the licking time compared with the control group (). This decrease occurred in a dose-dependent manner.
Table 2. Effect of citronellal (CTL) or indomethacin on formalin-induced pain.
Hot plate test
shows the results of the hot plate test. Two doses of CTL (100 and 200 mg/kg, i.p.) increased the latency time, in a dose-dependent manner, to the thermal stimulus in both the licking and jumping parameters evaluated. The reaction time parameter was only significantly increased in higher doses of CTL, which induced a nociceptive inhibition similar to the inhibition exerted by morphine (5 mg/kg, i.p.). Naloxone (0.5 mg/kg, i.p.) partially reversed the antinociceptive effect of the monoterpene.
Table 3. Antinociceptive effect of citronellal (CTL) in the hot plate test in the absence and presence of naloxone in mice.
Discussion
Citronellal (CTL) is a monoterpene reported to be a major component of the essential oils in various aromatic plant species (CitationLenardão et al., 2007; CitationQuintans-Júnior et al., 2008). Recently, various studies have shown that monoterpenes and their derivative compounds also exhibit several types of pharmacological properties, such as antinociception and sedation (Santos & Rao, Citation2000; CitationGaleotti et al., 2002; De Sousa et al., Citation2006a, Citation2007b). In this regard, the aim of the present study was to evaluate the CNS depressant properties and antinociceptive potential of CTL in mice.
Mice treated with CTL (50, 100, or 200 mg/kg, i.p.) presented behavioral alterations, such as decrease of spontaneous activity, palpebral ptosis, ataxia, analgesia, and sedation. These effects were dose-dependent and showed possible evidence that the effects on the CNS are similar to those of drugs that reduce central activity (CitationMorais et al., 2004; De Sousa et al., Citation2007a).
The potentiation of barbiturate action was investigated, and shows that CTL significantly altered (p < 0.05) the sleeping time. It is established that the potentiation of sleep time induced by pentobarbital must be a sedative or hypnotic action that is attributed to the involvement of central mechanisms in the regulation of sleep (CitationN’Gouemo et al., 1994; CitationAuzi et al., 2007) and involves potentiation of the γ-aminobutyric acid (GABA)ergic system (CitationSteinbach & Akk, 2001; CitationSivam et al., 2004).
Additionally, our results show that CTL produced dose-dependent inhibition of the inflammatory pain in mice, as determined by a significant reduction in acetic acid-induced abdominal writhing. Acetic acid-induced abdominal constriction is a standard, simple, and sensitive test for measuring analgesia induced by both opioids and peripherally acting analgesics (CitationHayes et al., 1987; CitationHunskaar & Hole, 1987). This test, besides being the most appropriate antinociceptive model for opioids (CitationHayes et al., 1987; CitationShaw et al., 1988), is also commonly employed as a visceral inflammatory pain model (CitationBarber & Gottschlich, 1992). In acetic acid-induced abdominal writhing, pain is elicited by the injection of an irritant such as acetic acid into the peritoneal cavity, which produces episodes of characteristic stretching (writhing) movements, and inhibition of the number of episodes by analgesics is easily quantifiable. Additionally, these results support the hypothesis of CTL participation in the inhibition of prostaglandin synthesis, as the nociceptive mechanism involves the process or release of arachidonic acid metabolites via cyclooxygenase (COX) and prostaglandin biosynthesis (CitationDuarte et al., 1988) during abdominal writhing induced by acetic acid.
The formalin test involves two phases: a neurogenic phase with release of substance P, and an inflammatory phase with release of serotonin, histamine, bradykinin, and prostaglandins (CitationMurray et al., 1988; CitationTjølsen et al., 1992). This is of interest, considering that both phases are sensitive to centrally acting drugs, such as opioids (CitationShibata et al., 1989). However, the second phase is also sensitive to NSAIDs (non-steroidal anti-inflammatory drugs) and corticosteroids (CitationHunskaar & Hole, 1987). In addition, it is generally agreed that N-methyl-d-aspartate (NMDA) receptors contribute to the persistent chemical stimulus during the late-phase central sensitization of dorsal horn neurons (wind-up; CitationHunter & Singh, 1994). Most drugs capable of reducing the excitability of spinal cord neurons, including opioids, NSAIDs, and glutamate antagonists, can also reduce or even abolish wind-up (CitationHerrero et al., 2000). shows that CTL (50, 100, and 200 mg/kg, i.p.) significantly inhibited (p < 0.05 and p < 0.01) the two phases.
The analgesic action presented by CTL involves supraspinal as well as spinal components, as demonstrated by utilization of the hot plate test (CitationYaksh & Rubi, 1976). The results suggest that CTL (100 and 200 mg/kg, i.p.) has a central analgesic effect, as evidenced by the prolonged delay in response time when mice were subjected to a nociceptive stimulus during a hot plate test. This central analgesic action was confirmed by the blocking effect of naloxone, a specific antagonist of morphinomimetic receptors (CitationBelvisi et al., 1998; CitationMundey et al., 2000).
It is concluded that CTL possesses CNS depressant and hypnotic properties. Additionally, CTL was effective as an anti-inflammatory and analgesic compound in various pain models, probably mediated via inhibition of prostaglandin synthesis as well as central inhibitory mechanisms (opioid system). Further studies currently in progress will enable us to understand the precise action mechanisms.
Declaration of interest
We would like to thank the National Council of Technological and Scientific Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico/CNPq/Brazil) and the Research Supporting Foundation of State of Sergipe (Fundação de Amparo à Pesquisa do Estado de Sergipe/FAPITEC-SE) for financial support. Two of the authors (Mônica S. Melo and Lais C. S. Sena) have scholarships from CNPq-UFS.
References
- Almeida RN, Navarro DS, Barbosa-Filho JM (2001): Plants with central analgesic activity. Phytomedicine 8: 310–322.
- Almeida RN, Falcão ACGM, Diniz RST, Quintans-Júnior LJ, Polari RM, Barbosa-Filho JM, Agra MF, Duarte JC, Ferreira CD, Antoniolli AR, Araújo CC (1999): Metodologia para avaliação de plantas com atividade no sistema nervoso central e alguns dados experimentais. Rev Bras Farm 80: 72–76.
- Auzi ARA, Hawisa NT, Sherif FM, Sarker SD (2007): Neuropharmacological properties of Launaea resedifolia. Braz J Pharmacog 17: 160–165.
- Barber A, Gottschlich R (1992): Opioid agonists and antagonists: an evaluation of their peripheral actions in inflammation. Med Res Rev 12: 525–562.
- Belvisi MG, Chung DM, Barnes PJ (1998): Opioid modulation of non-cholinergic neural bronchoconstriction in guinea-pig in vivo. Br J Pharmacol 95: 413–418.
- Broadbear JH, Negus SS, Butelman ER, Costa BR, Woods JH (1994): Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on κ-opioid agonists in mouse writhing assay. Psychopharmacology 15: 311–319.
- Carlini EA (2003): Plants and the central nervous system. Pharmacol Biochem Behav 3: 501–512.
- Carneiro MRG, Paumgartten FJR, Felzenswalb I (1997): Evaluation of the mutagenic potential of monoterpenoid compounds. Mutat Res 379(1): S111.
- De Sousa DP, Nóbrega FFF, Claudino FS, Almeida RN, Leite JR, Rita Mattei R (2007a): Pharmacological effects of the monoterpene α,β-epoxy-carvone. Braz J Pharmacog 17: 170–175.
- De Sousa DP, Júnior EVM, Oliveira FS, Almeida RN, Nunes XP, Barbosa-Filho JM (2007b): Antinociceptive activity of strutural analogues of rotundifolone: structure-activity relationship. Z Naturforsch C 62: 39–42.
- De Sousa DP, Quintans-Júnior LJ, Almeida RN (2007c): Evolution of the anticonvulsant activity of α-terpineol. Pharm Biol 45: 69–70.
- De Sousa DP, Oliveira FS, Almeida RN (2006a): Evaluation of the central activity of hydroxydihydrocarvone. Biol Pharm Bull 29: 811–812.
- De Sousa DP, Schefer RR, Brocksom U, Brocksom TJ (2006b): Synthesis and antidepressant evaluation of three parabenzoquinone mono-oximes and their oxy derivatives. Molecules 11: 148–155.
- De Sousa DP, Gonçalves JCR, Quintans-Júnior LJ, Cruz JS, Araújo DAM, Almeida RN (2006c): Study of anticonvulsant effect of citronellol, a monoterpene alcohol, in rodents. Neurosci Lett 401: 231–235.
- Duarte IDG, Nakamura M, Ferreira SH (1988): Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res 21: 341–343.
- Elisabetsky E, Coelho-De-Souza GP, Santos MAC, Siqueira IR, Amador TA (1995): Sedative properties of linalool. Fitoterapia 66: 407–414.
- Galeotti N, Mannelli LD, Mazzanti G, Bartolini A, Ghelardini C (2002): Menthol: a natural analgesic compound. Neurosci Lett 322: 145–148.
- Hayes AG, Sheehan MJ, Tyers TB (1987): Differential sensitivity of models of antinociception in the rat, mouse and guinea-pig to μ- and κ-opioid receptor agonists. Br J Pharmacol 91: 823–832.
- Herrero JF, Laird JM, López-Garcia JA (2000): Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol 61: 169–203.
- Hunskaar S, Hole K (1987): The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30: 103–104.
- Hunter JC, Singh L (1994): Role of excitatory amino acid receptors in the mediation of the nociceptive response to formalin in the rat. Neurosci Lett 174: 217–221.
- Jacob JJC, Tremblay EC, Coiomeel MC (1974): Facilitation of nociceptive reactions by naloxone in mice and rats. Psychopharmacology 37: 213–223.
- Jacob JJC, Ramabadran K (1978): Enhancement of a nociceptive reaction by opiate antagonists in mice. Br J Pharmacol 64: 91–98.
- Koster R, Anderson M, Beer EJ (1959): Acetic acid for analgesic screening. Fed Proc 18: 412–416.
- Lawless J (2002): The Encyclopedia of Essential Oils. London, Thorsons, p. 84.
- Lenardão EJ, Botteselle GV, Azambuja F, Perin G, Jacob RG (2007): Citronellal as key compound in organic synthesis. Tetrahedron 63: 6671–6712.
- Morais LC, Quintans-Júnior LJ, Franco CI, Almeida JR, Almeida RN (2004): Antiparkinsonian-like effects of Plumbago scandens on tremorine-induced tremors methodology. Pharmacol Biochem Behav 79: 745–749.
- Mundey MK, Ali A, Mason R, Wilson VG (2000): Pharmacological examination of contractile responses of the guinea-pig isolated ileum produced by opioid receptor antagonists in the presence of, and following exposure to, morphine. Br J Pharmacol 131: 893–902.
- Murray CW, Porreca F, Cowan A (1988): Methodological refinements in the mouse paw formalin test: an animal model of tonic pain. J Pharmacol Methods 20: 175–186.
- N’Gouemo P, Nquemby-Bina C, Baldy-Moulinier M (1994): Some neuropharmacological effects of an ethanol extract of Maprounea africana in rodents. J Ethnopharmacol 43: 161–166.
- Phillipson JD (2001): Phytochemistry and medicinal plants. Phytochemistry 56: 237–243.
- Quintans-Júnior LJ, Souza TT, Leite BS, Lessa NMN, Bonjardim LR, Santos MRV, Alves PB, Blank AF, Antoniolli AR (2008): Phytochemical screening and anticonvulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in rodents. Phytomedicine 15: 619–624.
- Reanmongkol W, Matsumoto K, Watanabe H, Subhadhirasakul S, Sakai SI (1994): Antinociceptive and antipyretic effects of alkaloids extracted from the stem bark of Hunteria zeylanica. Biol Pharm Bull 17: 1345–1350.
- Santos FA, Rao VSN (2000): Antiinflamatory and antinociceptive effects of 1,8-cineole, a terpenoid oxide present in many plant essential oils. Phytother Res 14: 240–244.
- Shaw JS, Rourke JD, Burns KM (1988): Differential sensitivity of antinociceptive tests to opioid agonists and partial agonists. Br J Pharmacol 95: 578–584.
- Shibata M, Ohkubo T, Takahashi H, Inoki R (1989): Modified formalin test: characteristic biphasic pain response. Pain 38: 347–352.
- Silva MIG, Neto MRA, Neto PFT, Moura BA, Amaral JF, De Sousa DP, Vasconcelos SMM, Sousa FCF (2007): Central nervous system activity of acute administration of isopulegol. Pharmacol Biochem Behav 88: 141–147.
- Sivam SP, Nabeshima T, Ho IK (2004): Acute and chronic effects of pentobarbital in relation to postsynaptic GABA receptors: a study with muscimol. J Neurosci Res 7: 37–47.
- Steinbach JH, Akk G (2001): Modulation of GABAA receptor channel gating by pentobarbital. J Physiol 537: 715–733.
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992): The formalin test: an evaluation of the method. Pain 51: 5–17
- Yaksh TL, Rubi TA (1976): Analgesia mediated by a direct spinal action of narcotics. Science 192: 1357–1358.
- Viana GSD, Vale TG, Silva CMM, Matos FJD (2000): Anticonvulsant activity of essential oils and active principles from chemotypes of Lippia alba (MILL.) Ne Brown. Biol Pharm Bull 23: 1314–1317.