Abstract
The present study aims to evaluate the hepatoprotective activity of Stereospermum suaveolens DC (Bignoniaceae). Hepatoprotective activity is studied by carbon tetrachloride (CCl4)-induced liver damage in albino rats. The degree of protection in this activity has been measured by using biochemical parameters such as serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), alkaline phosphatase (ALP), total bilirubin, LDL-cholesterol and SOD, CAT, GSH, total thiols, NO, and lipid peroxidation in liver tissue homogenate. The results suggest that the methanol stem bark extract of Stereospermum suaveolens at the doses 125, 250, and 500 mg/kg and reference standard Liv-52 treated group produced significant (p <0.001) hepatoprotection against CCl4-induced liver damage by decreasing the activities of serum enzymes, bilirubin and lipid peroxidation. The extract significantly (p <0.001) increased levels of SOD, CAT, GSH and total thiols, as compared to control group. Histopathological studies further substantiate the protective effect of the extract. It was concluded that methanol stem bark extract of Stereospermum suaveolens showed effective hepatoprotective activity.
Introduction
Reactive oxygen species (ROS) are formed constantly in the human body and are removed by enzymatic and non-enzymatic oxidative defense systems. Many of these have been implicated in the pathology of various human diseases (CitationMitra et al., 1999). The liver plays a major role in detoxification and excretion of many endogenous and exogenous compounds (CitationReddy et al., 1993). In addition, human beings consume a lot of synthetic drugs during disease conditions, which are alien to the body organs, and may produce a variety of toxic manifestations. Management of liver diseases is still a challenge to modern medicine. It is therefore necessary to search for alternative drugs for the treatment of liver diseases to replace the current use of drugs of doubtful efficacy and safety (CitationRajesh & Latha, 2004). Ayurveda, the ancient system of Indian medicine, has cited various herbs were for the management of liver disorders. Many herbal formulations like Liv-52 (CitationGoel & Dhawan, 1991), HD-03 (CitationMitra et al., 1998) and Ocimum sanctum Linn. (Labiatae) (CitationChattopadhyay et al., 1992) are available for treating liver disorders. Similarly, flavonoids are phenolic compounds widely distributed in plants and have been reported to exert multiple biological effects including antioxidant and free radical scavenging abilities (CitationMiddleton et al., 2000).
Stereospermum suaveolens DC (Bignoniaceae); commonly known as “patala”; Trumpet is widely available in India. The plant contains lapachol, dinatin, β-sitosterol, saponins, and palmitic, stearic, and oleic acids (CitationChattarjee & Asma, 2000). It is widely used by traditional practitioners as an analgesic, antidyspeptic and liver stimulant, and to treat wound healing, asthma, and semen debility (CitationChattarjee & Asma, 1997). Preliminary phytochemical studies in our laboratory revealed the presence of flavonoids, alkaloids, and triterpenoids in stem bark methanol extract. The present study evaluates the hepatoprotective activity of Stereospermum suaveolens stem bark methanol extract.
Materials and methods
Chemicals and reagents
2-Thiobarbituric acid (TBA), trichloroacetic acid (TCA), 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), epinephrine, Griess reagent were obtained from Sigma-Aldrich (St. Louis, MO, USA) and serum glutamate oxaloacetate transaminase (GOT), glutamate pyravate transaminase (GPT), Alkaline phosphatase (ALP), total bilirubin, LDL-cholesterol test kits were obtained from Erba Diagnostic, Mallaustr, Germany. Carbon tetrachloride (Nice Chemicals, Cochin, India), standard Liv-52 tablets (Himalaya Drug Co., Bangalore). All other reagents were of analytical grade. Refrigerator centrifuge (MPW-350R), UV-Spectrophotometer (UV-1601) was from Shimadzu, Kyoto, Japan
Plant material and preparation of extract
Stereospermum suaveolens is widely available in India. The fresh stem barks was collected in the month of May-June, 2008. It was identified and authenticated by S. A. Kappali, Botanist, Department of Botany, Basaveshwar Science College, Bagalkot, Karnataka. A voucher specimen (No: BSC/Pharmacy/2008/1/11) was deposited in the department for future reference. The cleaned and air-dried barks were subjected to coarse powdering and passed through a 44 mesh sieve to get a uniform size. The powder was extracted with petroleum ether to de-fat and then by methanol (60°-65°C) for 24 h by using Soxhlet apparatus, which yielded 0.34% brownish solid mass. The methanol extract was suspended and prepared using 5% Tween 80 and hepatoprotective activity studies against CCl4-induced hepatotoxicity were carried out.
Animals
Thirty six Wistar albino rats of either sex (220-250 g) were used for the study. Animals were procured from the National Institute of Nutrition, Hyderabad, and were kept in quarantine for 10 days under standard conditions (Temperature 22° ± 2°C; Relative humidity 55% ± 5%) for 12 h dark and 12 h light cycle respectively and are given standard pellet food (Hindustan Lever) and water ad libitum throughout the experimental period. The study was approved by the Institutional Animal Ethical Committee (IAEC-Clearance: 1-8/2007), H.S.K. College of Pharmacy, Bagalkot, Karnataka.
Acute toxicity study
Acute oral toxicity study was performed as per OECD-423 Guidelines. Female Swiss albino mice (20-25 g) were randomly distributed to six groups (n = 6). The animals were fasted overnight and methonol extract of Sterospermum suvaveolens was administered orally at the dose of 100, 200, 400, 800, 1600, and 3200 mg/kg. The animals were closely observed for 24 h for toxic symptoms and 72 h for mortality rate.
Hepatoprotective activity
The animals were divided into six groups of six animals each. The rats of group I served as normal, received normal saline 10 mL/kg; group II served as control. Rats of group I and II received the vehicle Normal saline only. Rats of group III served as positive control and received 56 mg/kg of Liv-52 and rats of groups IV, V and VI received 125, 250, and 500 mg/kg Stereospermum suaveolens methanol extract for a period of 19 days orally. On days 15, 17, and 19 the rats of groups II, III, IV, V and VI received 1 ml/kg of CCl4 in liquid paraffin (1:1) orally with the respective assigned treatments (CitationMitra et al., 1998)
Assessment of biochemical parameters
After the 24 h of the last dose of CCl4, blood was collected by retro-orbital plexus and centrifuged to separate serum for the estimation of SGOT, SGPT (CitationReitman & Frankel, 1957), ALP (CitationKind & King, 1954), total bilirubin (CitationJendrassic & Grof, 1938) and serum LDL-cholesterol (CitationAllain et al., 1952). Each rat was autopsied under light ether anesthesia. The isolated livers were perfused with 0.9% ice cold normal saline and 10% w/v liver homogenate was prepared with 0.1M phosphate buffer (pH 7.4) and centrifuged at 3000 rpm for 10 min at 4°C and supernant was stored immediately at -8°C for the further estimation of total protein (CitationLowery et al., 1951), lipid peroxidation (CitationPrabhakar et al., 2006; CitationBraughler et al., 1987), GSH (CitationPrabhakar et al., 2007), total thiols (CitationSedlak & Lindsy, 1968), SOD (CitationMisra & Fridovich, 1972), CAT (CitationClaiborne, 1985) and nitric oxide (CitationDawson & Dawson, 1995) levels in liver tissue.
Histopathological studies
Liver tissues were collected in 10% formalin for proper fixation. These tissues were processed and embedded in paraffin wax. Sections of 5-6 µm in thickness was cut and stained with hematoxylin and eosin dye, were observed microscopically for histopathological changes (Luna, 1996).
Statistical analysis
The data of biochemical parameters were expressed as mean ± SEM. Results were analyzed statistically using one way analysis of variance (ANOVA) followed by multiple Dunnett’s test. The minimum level of significance was fixed at p <0.05.
Results
The different doses of methanol extract of Stereospermum suaveolens shows significant hepatoprotective action. The results were summarized in and . At the end of 19 days treatment, blood samples of control group animals showed significant (p < 0.001) increase in the levels of serum biochemical parameters as compared to normal group animals, whereas blood samples from animals treated with different doses (125, 250, 500 mg/kg) of extract showed significant (p < 0.001 − p < 0.01) decrease in the levels of SGOT, SGPT, ALP, total bilirubin and LDL-cholesterol, when compared with control group. Similarly, the liver homogenate showed significant (p <0.001 – p < 0.05) reduced activities of SOD, CAT, GSH, total thiols and increased levels of NO and LPO were observed in the control group. The administration of different doses of methanol extract and standard Liv-52 treated group rats showed significant (p < 0.001) increase in SOD, CAT, GSH, total thiols level with decreased level of NO and LPO, as compared with the control group.
Table 1. Hepatoprotective activity of Stereospermum suaveolens against CCl4-induced hepatotoxicity in albino rats.
Table 2. Antioxidant activity of Stereospermum suaveolens against CCl4-induced hepatotoxicity.
Histopathological studies
Histopathological observations of the normal group rat liver (), showed normal hepatic architecture, absence of centrilobular necrosis and macrovesicular fatty changes and no dilation of portal vein. The control group rat liver (), exhibited intense centrilobular necrosis, vacuolization, macrovesicular fatty changes and distorted central vein architecture. The positive control group rat liver () also restores the normal histopathological observations. However, the different doses of extract 125 mg/kg treated () and 250 mg/kg treated () and 500 mg/kg treated groups () showed normal hepatic architecture, absence of centrilobular necrosis and macrovesicular fatty changes, and no dilation of portal vein except some vacuolization and dilation of hepatic vein in case of 500 mg/kg treated group rat liver.
Figure 1. Histopathological observations of normal group rat liver (A), showing normal hepatic architecture, absence of centrilobular necrosis and macrovesicular fatty changes, and no dilation of portal vein. The control (CCl4-treated) group rat liver (B), exhibited intense centrilobular necrosis, vacuolization, macrovesicular fatty changes and distorted central vein architecture. The positive control group rat liver (C) also restores the normal histopathological observations.
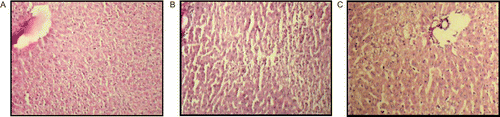
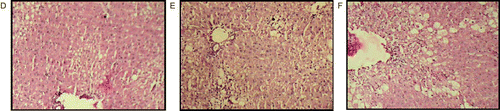
Figure 2. Histopathological observations of dose125 mg/kg treated group (D), 250 mg/kg treated group (E) and 500 mg/kg of extract-treated groups showed almost normal hepatic architecture, absence of centrilobular necrosis and macrovesicular fatty changes and no dilation of portal vein, but high dose (500 mg/kg) treated group showed some vacuolization and dilation of hepatic vein.
Discussion
The Stereospermum suaveolens methanol extracts showed potential capable of reducing CCl4-induced oxidative stress. The liver damage in CCl4-induced hepatotoxin is mainly assessed by determining the serum and tissue enzyme levels. Liver is considered to be highly sensitive to toxic agents. CCl4 administration causes necrosis or membrane damage of liver, thereby releasing enzymes into circulation (CitationButler, 1961). Liver cell injury induced by CCl4 involves the biotransformation of the toxin CCl4 by cytochrome P-450 to produce the trichloromethyl (CCl3.) free radicals, which causes peroxidative degradation in the adipose tissue, resulting in fatty infiltration of the hepatocytes. Trichloromethyl free radicals elicit lipid peroxidation of membrane in the presence of oxygen, generated by metabolic leakage from mitochondria. All these events culminate in the loss of integrity of the cell membrane and damage of hepatic tissues. Hepatocellular necrosis leads to elevation of serum biochemical markers such as SGOT, SGPT, ALP, total bilirubin, and LDL-cholesterol, which are released from the liver into the blood stream (CitationMitra et al., 1998). Accordingly the assessment of the level of enzyme markers, SGOT, SGPT, ALP are more specific to the liver and are better parameters for detecting liver injury (CitationChandrashekhar et al., 2004; CitationHewawasan et al., 2004).
Similarly, in CCl4-induced hepatotoxicity, the balance between ROS production and antioxidant defense may be lost, resulting in oxidative stress, in which a series of events deregulates the cellular functions leading to hepatic necrosis (CitationSanmugapriya & Venkataraman, 2006). The reduced activities of superoxide dismutase, catalase, glutathione, total thiols and increased lipid peroxidation in the CCl4-treated group rats. The methanol extract of Stereospermum suaveolens treatment animals showed significant increased level of superoxide dismutase, catalase, and reduced lipid peroxidation, which may be due to its free radical sacenging activity of extract and the increase in hepatic glutathione and total thiol levels by de novo glutathione and total thiol synthesis. Hepatocellular effect of CCl4 is due to oxidative damage by free radical generation, and antioxidant property is claimed to be one of the mechanisms of hepatoprotective drugs. CCl4 administration causes changes in liver enzyme markers, this leads to the damage of liver parenchymal cells (CitationSingh, 1980). Comparative histopathological study of the liver tissue from different groups of rats also confirmed the hepatotoxic level of CCl4. This study also confirms the preventive effect of Stereospermum suaveolens methanol extract which causes regeneration of hepatic cells and decreased necrosis.
Phytoconstituent flavonoids, triterpinoids, saponins and alkaloids are known to possess hepatoprotective activity (CitationManjunath, 2006). The preliminary phytochemical investigations of methanol stem bark extract of Stereospermum suaveolens were found to contain alkaloids, phenols, saponins, flavonoids and tannins. These antioxidant constituents present in methanol extract might be responsible for the free radical scavenging activity, inhibition of lipid peroxidation. Further work is necessary to isolate active principles and elucidate the actual mechanism involved in the hepatoprotective and antioxidant activity of this plant.
Acknowledgement
We are grateful to Principal B.V.V. Sangha, H.S.K. College of Pharmacy, Bagalkot, Karnataka for providing the facilities necessary to carry out the research work.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Allain CC, Bloor WR, Pelkhal F (1952): Estimation of serum cholesterol by colorimetric method. J Biol Chem 52: 191-214.
- Braughler JM, Chase RL, Pregenzer JF (1987): Oxidation of ferrous iron during peroxidation of various lipid substrates. Biochim Biophys Acta 921: 457–464.
- Butler TC (1961): Reduction of CCl4 in vivo and reduction of CCl4 and CHCl3 in vitro by tissue and tissue constituents. J Pharm Exp Ther 134: 311–318.
- Chandrashekhar VM, Abdul Haseeb TM, Habbu PV, Nagappa AN (2004): Hepatoprotective activity of Wrightia tinctoria ROXB in rats. Ind Drugs 41: 366–370.
- Chattarjee, Asma, Chandra, Prakashi, Satyesh (1997): The Treatise on Indian Medicinal Plants, Vol 5. New Delhi, National Institute of Science Communication, pp. 46–47.
- Chattarjee A, Chandra Prakashi S (2000): The Treatise on Indian Medicinal Plants, Vol 5. New Delhi, National Institute of Science Communication, pp. 10–11.
- Chattopadhyay RR, Sarkar SS, Ganguly S (1992): Hepatoprotective activity of Ocimum sanctum leaf extract against paracetamol induced hepatic damage in rats. Ind J Pharmacol 24: 163–165.
- Claiborne L (1985): Handbook of Methods or Oxygen Radical Research. London, CRC Press, pp. 22.
- Dawson TM, Dawson VL (1995): Nitric oxide: Actions and pathological roles. The Neuroscientist 1: 7–18.
- Goel A, Dhawan DK (1991): Influence of Liv-52 on carbon tetrachloride-induced hepatotoxicity: A biochemical study. Ind J Pharmacol 23: 182–184.
- Hewawasam RP, Jayatilaka KAPW, Pathirana C, Mudduwa LKB (2004): Hepatoprotective effect of Epaltes divaricata extract on carbon tetrachloride induced hepatotoxicity in mice. Ind J Med Res 120: 30–34.
- Jendrassic L, Grof P (1938): Colorimetric estimation of bilirubin. Biochemistry 81: 297-314.
- Kind PRN, King EJ (1954): Estimation of plasma phosphatase by determination of hydrolysed phenol with aminoantipyrine. J Clin Path 7: 322-354.
- Lowery OH, Rosenbrough NJ, Farr AL, Randall RJ (1951): Protein measurement with Folin-phenol reagent. J Biol Chem 193: 265–275.
- Luna LG (1966). Manual of Histological Staining. London, Methods of the Armed Forces Institute of Pathology, pp. 1–31.
- Manjunath BK (2006): Hepatoprotective activity of Pterocarpus santhalinus L.f., an endangered medicinal plant. Ind J Pharmacol 38: 25–28.
- Middleton E, Jr Kandaswami, C, Theoharides TC (2000): The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev 52: 673–751.
- Misra HP, Fridovich I (1972): The role of superoxide anion in the autooxidation of epinephrine and a sample assay for superoxide dismutase. J Biol Chem 247: 3170–3175.
- Mitra SK, Venkataranganna MV, Sundaram R, Gopumadhavan S (1998): Effect of HD-03, a herbal formulation, on the antioxidant defense system in rats. Phytother Res 12: 114–117.
- Mitra SK, Venkataranganna MV, Sundaram R, Gopumadhavan S (1999): Antioxidant activity of AO-8, a herbal formulation in vitro and in vivo experimental models. Phytother Res 13: 300–303.
- Prabhakar KR, Veerapur VP, Kumar V, Priyadarsin KI, Rao BSS, Unnikrishnan MK (2006): Evaluation and optimization of radio protective activity of Coronopus didymus Linn. in γ-irradiated mice. Int J Rad Biol 82: 1–12.
- Prabhakar KR, Veerapur VP, Bansal P, Vipan KP, Machendar Reddy K, Kumar B, Priyadarsini KI, Unnikrishnan MK (2007): Antioxidant and radioprotective effect of the active fraction of Pilea microphylla (L) ethanolic extract. Chemico-Biol Interact 165: 22–32.
- Rajesh MG, Latha MS (2004): Protective activity of Glycyrrhiza glabra Linn. on carbon tetrachloride-induced peroxidative damage. Ind J Pharmacol 36: 284–287.
- Reddy PB, Reddy CK, Rambhau D, Venkateshvaralu V, Murthy VN (1993): Antihepatotoxic activity of some Ayurvedic preparations. Ind J Pharm Sci 55: 137–140.
- Reitman S, Frankel S (1957): A colorimetric method for the determination of serum glutamic oxaloacetate and glutamic pyruvic transaminase. Am J Clin Path 28: 56–63.
- Sanmugapriya E, Venkataraman S (2006): Studies on hepatoprotective and antioxidant actions of Strychnos potatorum Linn. seeds on CCl4-induced acute hepatic injury in experimental rats. J Ethnopharmacol 105: 154–160.
- Sedlak J, Lindsy R (1968): Estimation of total, protein bound, and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Analy Biochem 25:192–205.
- Singh ID (1980): In:Talwar GP., ed., Chemical and Environmental toxicity. Textbook of Biochemistry and Human Biology. , New Delhi, Prentice Hall of India. 241–267.
