Abstract
Aqueous extract of Bulbine natalensis Baker (Asphodelaceae) stem at 25, 50 and 100 mg/kg body weight was investigated for anabolic and androgenic effects in male Wistar rats. Sixty male rats were grouped into four (A-D) consisting of 15 each. Group A (control) was orally treated with 0.5 mL of distilled water for 14 days while groups B, C and D were treated like the control except they received 0.5 mL containing 25, 50, and 100 mg/kg body weight of the extract respectively. All the doses of the extract increased (P <0.05) the testicular-body weight ratio as well as alkaline phosphatase activity, glycogen, sialic acid, protein, and cholesterol content of the testes except the single administration of 100 mg/kg body weight which compared well (P>0.05) with the controls for glycogen and cholesterol. The testicular and serum testosterone concentration were increased except in the 100 mg/kg body weight where the effect on the tissue and serum hormone did not manifest until after the first and seven daily doses respectively. Testicular acid phosphatase activity, serum follicle stimulating and luteinizing hormone concentrations also increased at all the doses except in the 100 mg/kg body weight where the effect on the enzyme and the hormone did not manifest until after seven days. The increases were most pronounced in the 50 mg/kg body weight extract treated animals. The results indicate anabolic and androgenic activities of Bulbine natalensis stem in male rat testes with the 50 mg/kg body weight of the extract exhibiting the highest anabolizing and androgenic acti vities. These activities further support the folkloric use of the plant most especially at 50 mg/kg body weight in the management of male sexual dysfunction in South Africa.
Introduction
In many countries of the world, the use of medicinal plants in the management of various ailments is increasing empirically. This may be due largely to perceived safety just because of their natural origin, easy availability, accessibility and scientific studies that are lending credence to their acclaimed use (CitationYakubu et al., 2007a). Several of these plants have been used to manage various diseases/inadequacies such as sexual dysfunction, diabetes, hypertension, fever and obesity. For example, the stem of Fadogia agrestis Schweinf. Ex Hiern (Rubiaceae) and the kernels of Terminalia catappa L. (Combretaceae) have been reported to exhibit aphrodisiac activity and thus could be explored in the management of sexual dysfunction in animals (CitationRatnasooriya & Dharmasiri, 2002; CitationYakubu et al., 2005). Many medicinal plants used as aphrodisiacs such as Hibiscus macranthus Hochst A ex Rich (Malvaceae), Basella alba Lam (Basellaceae), Piper guineense Schum & Thonn (Piperaceae), and Massularia acuminata G. Don Bullock ex Hoyl. (Rubiaceae) have also been reported to exhibit anabolizing and androgenic effects in male rats (CitationMainkar et al., 1986; CitationMoundipa et al., 1999; CitationYakubu et al., 2008). One plant that is yet to be investigated scientifically for anabolizing and androgenic activities despite its ethnomedicinal use as an aphrodisiac is Bulbine natalensis Baker (Asphodelaceae).
Bulbine natalensis, locally known as “ibhucu” (Zulu), “rooiwortel” (Afrikaans) and “ingcelwane” (Xhosa), is widely distributed in the eastern and northern parts of South Africa (CitationVan Wrk et al., 1997). The leaf sap is used in the management of wounds, burns, rashes, itches, ringworms, and cracked lips. An infusion of the roots is also taken to quell vomiting, diarrhea, convulsion, venereal diseases, diabetes and rheumatism (CitationPujol, 1990).
Phytochemical screening of the aqueous extract of Bulbine natalensis stem revealed the presence of tannins (0.481%), anthraquinones (0.152%), cardiac glycosides (0.887%), saponins (1.97%), and alkaloids (0.2%), while phenolics, flavonoids, steroids, phlobatannins, triterpenes, and caffeine were not detected (CitationYakubu & Afolayan, 2009). It has also been reported that the administration of the aqueous extract of the plant stem at 25 and 50 mg/kg body weight significantly increased penile erection indices, mount frequency, intromission frequency, ejaculatory frequency and post ejaculatory interval (CitationYakubu & Afolayan, 2009). These parameters which are controlled by androgens, have anabolic and androgenic effect on different body tissues (CitationMooradian et al., 1987).
Since B. natalensis has a positive impact on penile erection and sexual behaviour parameters which are dependent on androgen, the present study was undertaken to ascertain the androgenic and anabolizing activities of the aqueous extract of the plant stem on rat testes.
Materials and methods
Plant material and authentication
The plant samples, collected from a single population in the Eastern Cape in March, 2008 were authenticated by Prof. D.S. Grierson of the Department of Botany, University of Fort Hare, South Africa. A voucher specimen (Yakmed. 2008/1) was deposited at the Giffen Herbarium of the University.
Animals
Apparently healthy male albino rats (Rattus norvegicus), three months old, weighing 206 ± 4.67 g were obtained from the Animal House of the Agricultural and Rural Development Research Institute, University of Fort Hare. The animals were housed in clean aluminium cages placed in well-ventilated house conditions (temperature 23° ± 1°C; photoperiod: 12 h natural light and 12 h dark; humidity: 45–50%). They were also allowed free access to Balanced Trusty Chunks (Pioneer Foods, Huguenot, South Africa) and tap water. The cages were cleaned daily. The study was carried out following approval from the ethical committee of the University of Fort Hare on the use and care of experimental animals.
Assay kits and enzyme substrates
The assay kits for cholesterol, testosterone, luteinizing and follicle stimulating hormones were obtained from Roche Diagnostic, Mannheim, Germany, while 4-nitrophenyl phosphate (disodium salt) was a product of Sigma-Aldrich, St. Louis, USA. All other reagents used were of analytical grade and were supplied by Merck Chemicals, Bellville, South Africa.
Preparation of extract
The dried plant material was pulverized with an electric blender. The powder (20 g) was extracted in 1 L of distilled water for 48 h at room temperature with constant shaking on a Stuart Scientific Orbital Shaker (SO1, Stone, UK). The extract was filtered with Whatman (Maidstone, UK) No. 1 filter paper and the resulting filtrate was freeze-dried using a Vir Tis benchtop K (Vir Tis, Gardiner, NY) to give a yield of 4.71 ± 0.04 g. This was then reconstituted separately in distilled water to give the required doses used in this study.
Animal grouping and extract administration
Sixty male rats were completely randomized into four groups of 15 each and orally administered as follows: Group A (control) received 0.5 mL distilled water, while groups B, C and D were treated as the control except they received an equal volume of the extract containing 25, 50, and 100 mg/kg body weight respectively. The administration was done using a metal oropharyngeal cannula. Five rats from each of the groups were sacrificed 24 h after 1, 7, and 14 days of their respective daily doses.
Preparation of serum and testes homogenates
The procedure described by CitationYakubu et al. (2005) was employed in the preparation of serum. Briefly, under ether anesthesia, rats were made to bleed through their cut jugular veins which were slightly displaced (to prevent contamination of the blood by interstitial fluid) into clean, dry centrifuge tubes. The blood was left for 10 min at room temperature to clot. The tubes were then centrifuged at 1282 g × 5 min using a Hermle bench top centrifuge (Hermle, Z300, Hamburg, Germany). The sera were later aspirated with Pasteur pipettes into sample bottles and used for the hormonal assay within 12 h of preparation. The rats were thereafter quickly dissected in the cold, the testes excised and transferred into ice-cold 0.25 M sucrose solution. The organ was freed of fat and surrounding tissues, blotted with clean tissue paper and then weighed. The testes were then homogenized in ice-cold 0.25 M sucrose solution (1:5 w/v) (CitationAkanji & Yakubu, 2000). The homogenates were kept frozen overnight at -20˚C (to ensure maximum release of the enzymes and hormones located in the cells of the tissues) before being used for the various biochemical assays.
Determination of androgenic parameters
The concentration of serum hormones was determined using a Roche E170 modular analytics immunoassay analyzer (Roche Diagnostic, Mannheim, Germany) as described in the assay kit instruction manual for testosterone, follicle stimulating and luteinizing hormones (CitationWang et al., 1993; CitationSinha-Hikim et al., 1995) while those of protein, glycogen, cholesterol, sialic acid as well alkaline and acid phosphatase activities in the testes were determined using standard methods (CitationGornall et al., 1949; CitationKemp et al., 1954; CitationWarren, 1959; CitationFredrickson et al., 1967; CitationWright et al., 1972a, Citationb). The testes-body weight ratio was calculated using the expression of CitationYakubu et al. (2008).
Statistical analysis
Results were expressed as the mean of five replicates ± SD. Percentage data were arcsine transformed before analysis. Means were analyzed using a one-way ANOVA and values of P <0.05 were considered statistically significant (CitationMahajan, 1997). In all the Figures, bars carrying letters different from the control for each day are significantly different.
Results
The effects of administration of aqueous extract of Bulbine natalensis stem at 25, 50 and 100 mg/kg body weight on the anabolizing and androgenic parameters of male rat testes are depicted in and . The extract at all the doses increased (P <0.05) the testicular-body weight ratio (). By the end of the experimental period, the organ body weight had increased to 2.5, 2.9, and 2 fold of the control value in the 25, 50, and 100 mg/kg body weight extract treated animals, respectively. The highest increase in the testicular parameter was produced in the 50 mg/kg body weight treated animals following the 7 and 14 daily doses.
Figure 1. Effect of administration of aqueous extract of Bulbine natalensis stem on testicular–body weight ratio of rats.
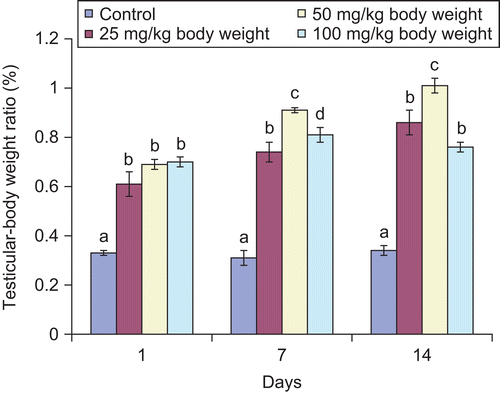
Figure 2. Effect of administration of aqueous extract of Bulbine nalalensis stem on testicular alkaline phosphatase activity.
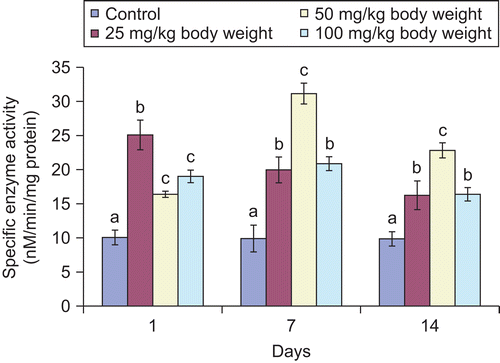
Figure 3. Effect of administration of aqueous extract of Bulbine nalalensis stem on testicular acid phosphatase activity of rats.
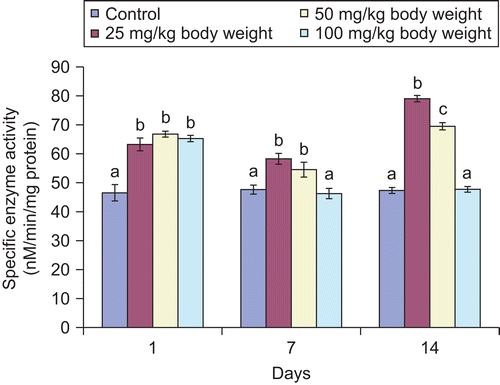
Figure 4. Effect of administration of aqueous extract of Bulbine nalalensis stem on rat testicular testosterone concentration.
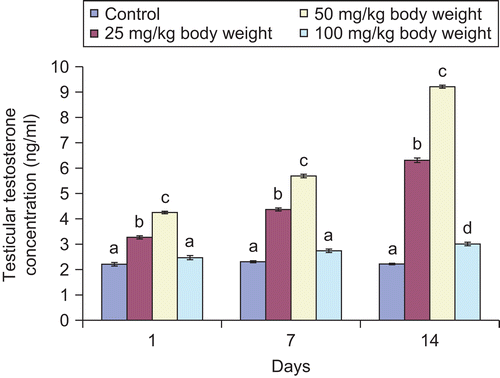
Figure 5. Effect of administration of aqueous extract of Bulbine nalalensis stem on male rat serum testosterone concentration.
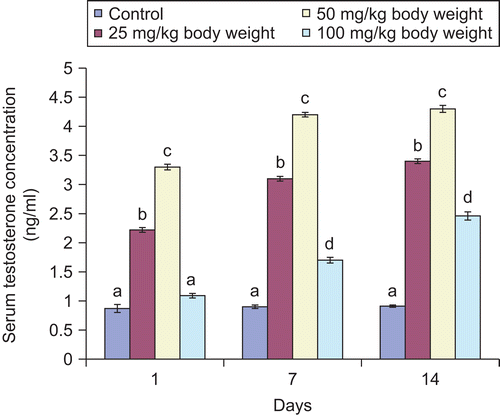
Figure 6. Effect of administration of aqueous extract of Bulbine nalalensis stem on male rat serum follicle stimulating hormone concentration.
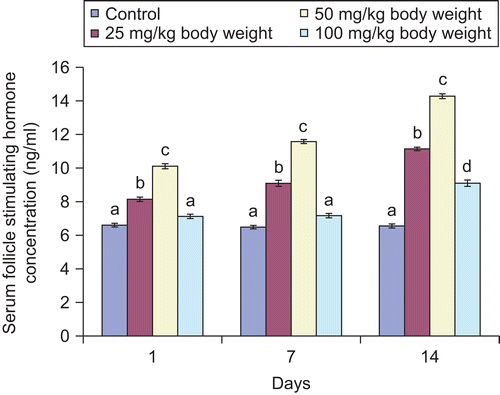
Figure 7. Effect of administration of aqueous extract of Bulbine nalalensis stem on male rat serum luteinizing hormone concentration.
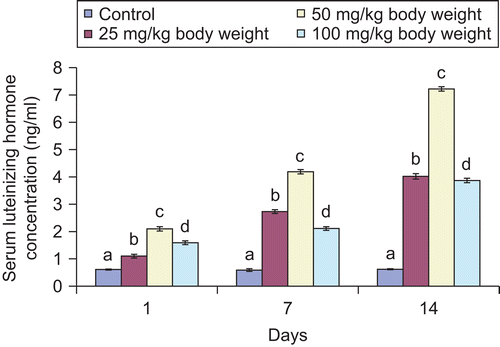
Table 1. Effect of administration of aqueous extract of Bulbine natalensis stem on some secretory constituents of male rat testes.
The activities of alkaline and acid phosphatase of the rat testes also increased significantly following the administration of the extract ( and ). The single administration of 25 mg/kg body weight produced the highest increase in the activity of alkaline phosphatase. Whereas the enzyme activity was highest at days 7 and 14 in the 50 mg/kg body weight of the extract treated animals, the alkaline phosphatase activity at 25 mg/kg body weight of the extract compared well with that of 100 mg/kg body weight at the other days of intervention (). Although, the activity of acid phosphatase increased at 25 and 50 mg/kg body weight throughout the period of administration, the increase produced by the 100 mg/kg body weight was not sustained beyond the single administration as values at the other days of intervention compared well with the control values ().
All the doses increased the testicular glycogen concentration throughout the experimental period except on the first day where the values compared well with the control (). The sialic acid and protein concentrations of the rat testes also increased in a manner that was not dose dependent. The increases in testicular protein by the single dose of the extract were lower than those at other days of intervention. The extract also elevated the levels of testicular cholesterol at all the doses except the single administration of the highest dose (100 mg/kg body weight) which compared well with the control value ().
Although the administration of the extract increased the testicular and serum testosterone concentration, such increase did not manifest in the 100 mg/kg body weight treated animals until after the seven daily doses for the tissue hormone and single dose for the serum hormone ( and ). The increase in the testicular and serum testosterone was most pronounced in the 50 mg/kg body weight treated animals.
All the doses of the extract except the 100 mg/kg body weight increased the concentration of serum follicle stimulating hormone throughout the experimental period. The increase in the serum hormone at the highest dose did not manifest until the 14th daily administration (). In contrast, the extract increased the concentration of luteinizing hormone in the serum of the animals throughout the period of administration. It is also noteworthy that the 50 mg/kg body weight of the extract produced the highest elevation of the serum hormone ().
Discussion
Evaluation of biochemical parameters such as testes–body weight ratio, concentrations of testicular secretory constituents like protein, cholesterol, sialic acid, glycogen, testosterone as well as alkaline and acid phosphatase activity, serum luteinizing and follicle stimulating hormones can give useful information on the androgenic and or anti-androgenic potential of plant extracts (CitationGupta et al., 2004; CitationWatcho et al., 2004; CitationYakubu et al., 2007b, Citation2008).
The increase in absolute testicular weight and the testes–body weight ratio observed with all the dose levels of B. natalensis stem in this study is probably a consequence of an increase in functional activity of the organ leading to increase in the secretory ability of the testes. Such increase in secretory activity of the organ, which is androgen dependent, was supported by the elevated levels of cholesterol, proteins, sialic acid and testosterone in the present study. This is an indication of the anabolizing and androgenic effects of the B. natalensis stem extract. This agreed with the findings of CitationWatcho et al. (2004) and CitationYakubu et al. (2008) following the administration of Mondia whitei Hook. F Skeels (Periplocaceae) roots and Massularia acuminata stem, respectively, to male rats.
Alkaline phosphatase is involved in the synthesis of nuclear proteins, nucleic acids and phospholipids, as well as the cleavage of phosphate esters. It also plays a role in mobilizing carbohydrates and lipid metabolites to be utilized either within the cells of the accessory sex structure or by the spermatozoa in the seminal fluid (CitationRamalingam & Vimaladevi, 2002). The elevated testicular alkaline phosphatase activity by the extract at all the doses suggests enhanced mobilization of carbohydrate and lipid metabolites which may be used by the accessory sex structure or the spermatozoa in the seminal fluid. The increase in the activity of the testicular enzyme in this study may be attributed to the saponin content of the extract since similar increase was observed by CitationWitthawaskul et al. (2003) following the administration of saponin mixture from the leaves of Schefflera leucantha Viguier (Araliaceae) to rats. The highest activity of alkaline phosphatase observed with the 50 mg/kg body weight of the extract further justifies the use of this dose in the folklore medicine of South Africa for the management of sexual inadequacies. In contrast, the alkaline phosphatase activity at the highest dose (100 mg/kg body weight) which compared well with the control may suggest non-androgenic activity at this dose.
Acid phosphatase is widely distributed in the testes and is important in the physiology of sperm (CitationBreton et al., 1996). Increased levels of the enzyme suggest anabolic effect of the extract. The absence of significant effect by the 100 mg/kg body of the extract on the acid phosphatase activity from seven days may be adduced to non-sensitivity to the synthetic mechanism of the enzyme by the bioactive principle in the extract at this dose (CitationWatcho et al., 2004).
The glycogen content of the testis, an index of energy storage, is directly proportional to the steroid hormones (CitationPathak & Prakash, 1989; CitationYakubu et al., 2007b). Sertoli cells and spermatogonia often contain glycogen where it provides a reserve of carbohydrates for seminiferous tubular cells. The increase in testicular glycogen after the single administration of the extract can be explained by an enhanced glycolysis during spermatogenesis (CitationGupta et al., 2005). Such increase in testicular glycogen may stimulate energy production that is essential for spermatogenic activity. This, however, contrasts the report of CitationGupta et al. (2005), where saponin content of the bark of Albizia lebbeck (L.) Benth (Mimosoideae) decreased glycogen and inhibited glycolysis during spermatogenesis. The lack of significant difference in the testicular glycogen concentration following the single administration of the extract could possibly suggest that the extract has not reached a sufficient level to start exerting any effect on the testicular parameter.
Testicular sialic acid content, which is androgen dependent, acts as a “lubricant” to facilitate the movement of sperm by reducing friction among the spermatozoa (CitationRiar et al., 1973). Structural integrity of the acrosomal membrane is also dependent on sialic acid content such that any alteration in its content may lead to changes in the motility and fertilizing ability of the sperm (CitationLevinsky et al., 1983). The elevated levels of testicular sialic acid content observed with B. natalensis stem in this study is an indication that the extract might have the ability to reduce friction among the spermatozoa. It may also suggest enhancement in the structural integrity of acrosomal membrane, which ultimately may stimulate the metabolism, motility and fertilizing capacity of spermatozoa (CitationChinoy & Bhattachary, 1997).
Testicular proteins are one of the constituents that ensure the maturation of the spermatozoa (CitationKasturi et al., 1995). Increased weight and high protein concentration of the testes indicates enhancement of testicular growth in addition to follicle stimulating hormone which is also essential for protein synthesis in the gonads (CitationMeans, 1975). The elevated levels of testicular protein observed with the extract in this study may be attributed to testosterone action (CitationGupta et al., 2004). Such increase in protein concentration which is an indication of anabolizing and androgenic effects of the extract may enhance sperm maturation (CitationYakubu et al., 2008). CitationWatcho et al. (2004) also observed similar increase in testicular protein content following the administration of M. whitei roots to male rats.
Cholesterol is the major substrate responsible for the anabolic effect of testosterone in males (CitationCarreau, 1996), and its requirement for normal testicular activity has been well established (CitationWatcho et al., 2004). The increase in testicular cholesterol concentration by the extract may be attributed to enhanced steroidogenesis. Such stimulation of steroidogenic pathway will lead to increased androgen concentration (CitationBedwall et al., 1994). The elevated levels of testicular cholesterol observed in this study which is also an indication of anabolic effect of the extract agreed with the findings of CitationKamtchouing et al. (2002) following the administration of aqueous extract of the rhizome of Zingiber officinale Roscoe (Zingiberaceae) and Pentadiplandra brazzeana Baill. (Pentadiplandraceae) root to male Wistar rats. CitationDixit and Gupta (1982) reported that solasodine, an alkaloid obtained from Solanum xanthocarpum Var. Jacquinis (Solanaceae) berries elevated testicular cholesterol in dog (Canis familiaris), therefore, the increase in the testicular lipid in this study may not be unconnected with the alkaloids in Bulbine natalensis stem.
Testosterone, luteinizing hormone (LH) and follicle stimulating hormone (FSH) are hormonal markers of androgenicity (CitationWalton et al., 1995). Testosterone, the principal circulating androgen, is secreted by Leydig cells under LH stimulation (CitationPayne, 1990). The main action of the hormone is to facilitate the maturation of round to elongated spermatids during spermiogenesis (CitationO’Donnell et al., 1994). It also stimulates the spermatid binding to Sertoli cells specifically at the transition from stages VII to VIII of the seminiferous epithelium (CitationCameron et al., 1994). The increase in testosterone concentration could possibly be due to an induction in the synthesis of the hormone by the Leydig cells, since the cells are the main source of testosterone in rats (CitationDe Krester, 1987). This implies that the extract stimulated the mechanism intervening in the process of the hormone synthesis in the Leydig cells (CitationMoundipa et al., 1999). The elevated testosterone content observed in this study might have resulted from the increase in cholesterol concentration since it is the starting material for androgen biosynthesis. A minimum level of blood androgen is required for the maintenance of body shape, muscle mass and strength, normal sexual desire, nocturnal penile tumescence, and non-erotic penile erections in most men. Therefore, the increase in testosterone by the extract, an indication of androgenic and anabolizing potential of the plant, may also be responsible for the enhanced male sexual behaviour reported by CitationYakubu and Afolayan (2009).
It is well known that gonadotropin (LH and FSH) secretion is basically controlled by the hypothalamic and testicular hormones (CitationGharib et al., 1990). Follicle stimulating hormone is required for the initiation and maintenance of spermatogenesis whereas luteinizing hormone stimulates the synthesis and secretion of testosterone. While GnRH stimulates, testosterone and its metabolites reciprocally depress FSH and LH secretion (CitationConn, 1986). Therefore, the elevated serum gonadotropins and testosterone may be explained by a decrease in the feed-back signal of steroid hormones on pituitary gonadotropins (CitationMalini et al., 1999). The elevated levels of luteinizing hormone by the extract might have enhanced the synthesis and secretion of testosterone as observed in this study. The pro-gonadotropic effect of the extract is an indication that it may support normal functioning of the Sertoli cells which may in turn enhance sperm cell maturation. The increases in the concentrations of the hormones imply direct effect on the testicular hormonogenesis. Gonadotropin sufficiency and testicular enhancement are indications of androgenicity. Therefore, the extract possesses anabolizing and androgenic potentials in male rat testes.
Several studies have shown that steroidal saponins contribute to increasing endogenous testosterone content by raising the levels of luteinizing hormones (CitationProtich et al., 1983; CitationGauthaman et al., 2002; CitationTemraz et al., 2006). The steroidal nature of saponins may facilitate its role as an intermediary in the steroidal pathway of androgen production. It could also act by binding to hormone receptors, which may result in conformational change that will enhance the physiological function of the hormones or bind to enzymes that are involved in the synthesis of such hormones and thus enhance its production (CitationGauthaman & Adaikan, 2008). CitationGauthaman et al. (2003) have also attributed the enhanced sexual behaviour parameters to the androgen increasing property of a steroidal saponin, protodioscin. Therefore, the raised levels of the hormones observed in this study could possibly be due to the saponin content of the extract.
Similarly, it has also been established that sex differentiation, growth and maintenance of the epididymis, prostate, seminal vesicle and testes are androgen-dependent processes (CitationAng et al., 2000). In rats, an elevated level of serum testosterone is associated with increased weight and enhanced secretory activity of the organs (CitationGonzales, 2001). Therefore, the increase in testes–body weight ratio, activity of alkaline and acid phosphatase, concentrations of testosterone, follicle stimulating and luteinizing hormones as well as other secretory metabolites of the testes indicates anabolizing and androgenic activities of B. natalensis stem. These activities could possibly be due to the presence of saponins and alkaloids in the aqueous extract of B. natalensis stem. The pronounced effect on these androgenic parameters by the 50 mg/kg body weight of the extract justifies the recommendation and the use of plant extract at this dose in the traditional medicine of South Africa for the management of sexual inadequacies in males.
Results obtained from this study further confirm the importance attached to Bulbine natalensis by traditional practitioner of South Africa for the management of male sexual dysfunction. This further lends credence to our previous study on the enhancement of male sexual behavior by the aqueous extract of Bulbine natalensis stem.
Declaration of interest
This research was supported with grants from Govan Mbeki Research and Development Centre, University of Fort Hare, and the National Research Foundation, South Africa. The authors are also grateful to the University of Ilorin, Nigeria for the Postdoctoral Fellowship support to Dr. M.T. Yakubu.
References
- Akanji MA, Yakubu MT (2000): α-Tocopherol protects against metabisulphite-induced tissue damage in rats. Nig J Biochem Mol Biol 15: 179–183.
- Ang HH, Cheang HS, Yusof AP (2000): Effect of Eurycoma longifolia Jack (Tongkat Ali) on the initiation of sexual performance of inexperienced castrated male rats. Exp Anim 49: 35–38.
- Bedwall RS, Edwards MS, Katoch M, Bahuguna A, Dewan R (1994): Histological and biochemical changes in testes of zinc deficient BALB/C strain of mice. Indian J Exp Biol 32: 243–247.
- Breton S, Smith PTS, Lui B, Brown D (1996): Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nature Med 2: 470–472.
- Cameron DF, Muffly KE, Nazian SJ (1994): Reduced testosterone during puberty results in a midspermiogenic lesion. Proc Soc Exp Biol Med 202: 457–464.
- Carreau S (1996): Paracrine control of human Leydig cell and Sertoli cell functions. Folia Histochem Cytol 34: 111–119.
- Chinoy NJ, Bhattachary S (1997): Effect of chronic administration of aluminium chloride on reproductive functions of the testes and some accessory sex organs of male mice. Indian J Environ Toxicol 7: 12–15.
- Conn PM (1986): The molecular basis of gonadotropin releasing hormone action. Endocr Rev 7: 3–10.
- De Krester DM (1987): The testis, in: Austin, CR, Short RV, eds, A Hormonal Control of Reproduction. New York, Academic Press, pp. 76–90.
- Dixit VP, Gupta RS (1982): Antispermatogenic/antiandrogenic properties of solasodine (C27H43O2N) obtained from Solanum xanthocarpum berries on the male genital tract of dog (Canis familiaris). A histophysiological approach. Int J Androl 5: 295–307.
- Fredrickson DS, Levy RI, Lees RS (1967): Fat transport in lipoproteins–An integrated approach to mechanisms and disorders. N Eng J Med 276: 148–156.
- Gauthaman K, Adaikan PG (2008): The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction-an evaluation using primates, rabbits and rats. Phytomedicine 15: 44–54.
- Gauthaman K, Adaikan PG, Prasada RNV (2002): Aphrodisiac properties of Tribulus terrestris extract (Protodioscin) in normal and castrated rats. Life Sci 71: 1385–1396.
- Gauthaman K, Adaikan PG, Prasada RNV (2003): Sexual effects of Puncturevine (Tribulus terrestris) extract (Protodioscin): An evaluation using a rat model. J Altern & Comp Med 9: 257–265.
- Gharib SD, Wierman ME, Shupnik MA, Chin WW (1990): Molecular biology of the pituitary gonadotropins. Endocr Rev 11: 177–197.
- Gonzales GF (2001): Function of seminal vesicles and their roles in male fertility. Asian J Androl 3: 251–258.
- Gornall AC, Bardawill CJ, David M.M (1949): Determination of serum protein by means of biuret reaction. J Biol Chem 177: 751–756.
- Gupta RS, Chaudhary R, Yadav RK, Verma SK, Dobhal MP (2005): Effect of saponins of Albizia lebbeck (L.) Benth bark on the reproductive system of male albino rats. J Ethnopharmacol 96: 31–36.
- Gupta RS, Kachhawa JB, Chaudhary R (2004): Antifertility effects of methanolic pod extract of Albizzia lebbeck. Asian J Androl 6: 155–159.
- Kamtchouing P, Mbongue-Fandio GY, Dimo T, Jatsa HB (2002): Evaluation of androgenic activity of Zingiber officinale and Pentadiplandra brazzeana in male rats. Asian J Androl 4: 299–301.
- Kasturi M, Manivannan B, Ahmed NR, Shaikh PD, Pattan KM (1995): Changes in epididymal structure and function of albino rat treated with Azardirachta indica leaves. Indian J Expt Biol 33: 725–729.
- Kemp A, Adrienne JM, Heijningen KV (1954): A colorimetric micro-method for the determination of glycogen in tissues. Biochem J 56: 646–648.
- Levinsky H, Sirger R, Bornet M, Sagiv M, Allolouf D (1983): Sialic acid content of human spermatozoa and seminal plasma in relation of sperm counts. Arch Androl 10:45–48.
- Mahajan BK (1997): Significance of difference in means, in: Mahajan BK, ed., Methods in Biostatistics for Medical and Research Workers. New Delhi, JAYPEE Brothers Medical Publishers, pp. 130–155.
- Mainkar AV, Naik VR, Dhuno VG, Aghsikar NV (1986): Anabolic activity of alcoholic extract of Cucumis trigonus roxburghii. Indian J Pharmacol 180: 261–262.
- Malini T, Manimaran RR, Arunakaran J, Aruldhas MM, Govindarajulu P (1999): Effect of piperine on testis of albino rats. J Ethnopharmacol 64: 219–225.
- Means AR (1975): Biochemical effects of follicle stimulating hormones on the testis, in: Greep RO, Ashwood EB, eds, Handbook of Physiology and Endocrinology. Washington DC, American Physiological Society, pp. 203–219.
- Mooradian AD, Moodley JE, Korenman SC (1987): Biological action of androgens. Endocrinol Rev 8: 11–28.
- Moundipa FP, Kamtchouing P, Koueta N, Tantchou J, Foyang NPR, Mbiapo FT (1999): Effect of aqueous extract of Hibiscus macranthus and Basella alba in mature rat testes function. J Ethnopharmacol 65: 133–139.
- O’Donnell L, McLachlan RI, Wreford NG, Robertson DM (1994): Testosterone promotes the conversion of round spermatids between stages VII and VIII of the rat spermatogenic cycle. Endocrinol 135: 2608–2614.
- Pathak S, Prakash AO (1989): Post-coital contraceptive effect of Ferula jaeschkeana Vatke. and its hormonal properties. Phytother Res 3: 61–66.
- Payne AH (1990): Hormonal regulation of cytochrome P450 enzymes, cholesterol sidechain cleavage and 17α-hydroxylase/C17-20 lyase in Leydig cells. Biol Reprod 42: 399–404.
- Protich M, Tsvetkov D, Nalbanski B, Stanislavov R, Katsarova M (1983): Clinical trial of a tribestan preparation in infertile men. Akush Ginekol (Sofia) 22: 326–329.
- Pujol J (1990): Naturafrica – the Herbalist Handbook. African Flora, Medicinal Plants. Durban, Jean Pujol Natural Healers’ Foundation, pp. 25–28.
- Ramalingam V, Vimaladevi V (2002): Effect of mercuric chloride on membrane-bound enzymes in rat testis. Asian J Androl 4: 309–311.
- Ratnasooriya WD, Dharmasiri MG (2002): Effect of Terminalia catappa seeds on sexual behaviour and fertility of male rats. Asian J Androl 2: 213–219.
- Riar SS, Setty BS, Kar AB (1973): Studies on the physiology and biochemistry of mammalian epididymis: Biochemical composition of epididymis. A comparative study. Fertil Steril 24: 353–362.
- Sinha-Hikim AP, Wang C, Leung A, Swerdloff RS (1995): Involvement of apoptosis in the induction of germ cell degeneration in adult rats after gonadotropin-releasing hormone against treatment. Endocrinology 136: 2770–2775.
- Temraz A, El Gindi OD, Kady HA, De Tommasi N, Braca A (2006): Steroidal saponins from the aerial parts of Tribulus alatus Del. Phytochemistry 67: 1011–1018.
- Van Wrk BE, Van Oudtshoorn B, Gericke N (1997): Medicinal Plants of South Africa. Pretoria, South Africa, Briza Publications, pp. 64–65.
- Walton S, Cunliffe WJ, Kaczkes K, Early AS, McGarrigle HHG, Katz M, Reese RA (1995): Clinical, ultrasound and hormonal markers of androgenicity in acne vulgaris. Br J Dermatol 133: 249–253.
- Wang C, Leung A, Sinha-Hikim AP (1993): Reproductive aging in the male Brown-Norway rat: A model for the human. Endocrinology 133: 2773–2781.
- Warren L (1959): The thiobarbituric acid assay of sialic acids. J Biol Chem 234: 1971–1975.
- Watcho P, Kamtchouing P, Sokeng SD, Moundipa PF, Tantchou J, Essame JL, Koueta N (2004): Androgenic effect of Mondia whitei roots in male rats. Asian J Androl 6: 269–272.
- Witthawaskul P, Panthong A, Kanjanapothi D, Taesothikul T, Lertprasertsuke N (2003): Acute and subacute toxicities of the saponin mixture isolated from Schefflera leucantha Viguier. J Ethnopharmacol 89: 115–121.
- Wright PJ, Leathwood PD, Plummer DT (1972a): Enzymes in rat urine. Alkaline phosphatase. Enzymologia 42: 317–327.
- Wright PJ, Leathwood PD, Plummer DT (1972b): Enzymes in rat urine. Acid phosphatase. Enzymologia 42: 459–462.
- Yakubu MT, Afolayan AJ (2009): Effect of aqueous extract of Bulbine natalensis (Baker) stem on the sexual behaviour of male rats. Int J Androl (in press) [Epub ahead of print] doi:10.1111/j. 1365-2605. 2008. 00910.x
- Yakubu MT, Akanji MA, Oladiji AT (2005): Aphrodisiac potentials of aqueous extract of Fadogia agrestis (Schweinf. ex Heirn) stem in male albino rats. Asian J Androl 7: 399–404.
- Yakubu MT, Akanji MA, Oladiji AT (2007a): Male sexual dysfunction and methods used in assessing medicinal plants with aphrodisiac potentials. Pharmacog Rev 1: 49–56.
- Yakubu MT, Akanji MA, Oladiji AT (2007b): Evaluation of antiandrogenic potential of aqueous extract of Chromolaena odoratum (L.) K. R. leaves in male rats. Andrologia 39: 235–243.
- Yakubu MT, Akanji MA, Oladiji AT, Adesokan AA (2008): Androgenic potentials of aqueous extract of Massularia acuminata (G. Don) Bullock ex Hoyl. stem in male Wistar rats. J Ethnopharmacol 118: 508–513.
