Abstract
The root extract of Hemidesmus indicus (Linn.) R. Br. (Asclepiadaceae) (HI) was studied for its cardioprotective effect in Langendorff-perfused rat hearts. HI was perfused for 15 min at a concentration of 0.09 g/L prior to 30 min global ischemia/120 min reperfusion (I/R). Recovery of functional parameters, reperfusion arrhythmias, and infarct size (TTC staining) served as the end-points. After 15 min of perfusion with HI, the left ventricular developed pressure (LVdevP) and HR (heart rate) were not altered significantly (p > 0.05), as compared with the pre-drug values. During R, HI showed a significantly higher (p < 0.05) recovery of LVdevP at nearly all time points. The recovery of maximal rate of pressure development (+dP/dtmax) and left ventricular end-diastolic pressure (LVEDP) at 40 min of R were significantly better than in non-treated controls. There was also a significant reduction in the total number of ventricular premature beats (VPB) and duration of ventricular tachycardia (VT). HI can protect ischemic myocardium against contractile dysfunction and reperfusion-induced arrhythmias and reduce the extent of irreversible tissue damage following I/R in rat hearts.
Introduction
Hemidesmus indicus (Linn.) R. Br. (Asclepiadaceae) (HI) is a twining shrub used in folk medicine and as an ingredient in Ayurvedic and Unani preparations. It is known as Indian sarsaparilla (English), Sugandhi, Anantamul (Sanskrit), Magrabu (Hindi), Ushbahindi (Persian), and Irimusk (Sinhalese). The plant has been used traditionally for the treatment of dyspepsia, blood tumors, cough, ulceration, leukorrhea, fever, rheumatoid arthritis, skin diseases, syphilis, urinary disorders, loss of appetite, scorpion and snake bites, etc. (CitationAnjaria et al., 2002). HI has been reported to have various effects such as hypoglycemic (CitationMurshed et al., 2005), hypolipidemic (CitationBopanna et al., 1997), antioxidant, antithrombotic (CitationMary et al., 2003), antiulcerogenic (CitationAnoop & Jegadeesan, 2003), anti-inflammatory, antipyretic (CitationLakshman et al., 2006), and hepatoprotective (CitationPrabakan et al., 2000). It mainly consists of hemidesmine, hemidesmol, hemidesterol, stearoptin, pregnane glycosides, β-sitosterol, indicusin, coumarin, volatile oils, saponins, triterpines, flavonoids, tannins, etc. (CitationAnjaria et al., 2002; CitationAnoop & Jegadeesan, 2003).
However, untill the present, no research work has been undertaken to study the cardioprotective effects of HI. Hence, this study was designed to evaluate the potential protective effect of HI on the model of ischemia–reperfusion (I/R) injury in the isolated perfused rat heart.
Materials and methods
Preparation of the extract
The standardized dry extract of HI, prepared as stated below, was kindly provided by Rumi Herbal Research Institute Private Limited, Chennai, India. In brief, dried roots of HI were coarsely powdered and refluxed with 50% ethanol by a hot percolation method and extracted. The yield was 23.18% of black-brown extractives containing 2.87 mg% of saponins and 1.62 mg% of tannins; heavy metals, arsenic and lead, were not more than 1 ppm; Escherichia coli and Salmonella were absent. The 600 mg dry extract was added to 15 mL of boiling distilled water. After 2 min boiling, the decoction was cooled and centrifuged to separate any undissolved material and the supernatant was considered as stock solution, containing 40 g/L of HI extract (CitationSuchalatha & Devi, 2004). The extract of HI was added to Krebs–Henseleit buffer (KHB) at a concentration of 0.09 g/L. The stock solution was prepared fresh, daily.
Experimental protocol
All the animals and experiments were handled as per European animal ethics guidelines. The rats were randomly divided into control (C) and drug-treated groups (HI). The hearts were allowed to stabilize (15 min) before further interventions. After stabilization, hearts of the HI group were perfused with extract (0.09 g/L) for 15 min, while those of the C group were perfused with KHB. Hearts of both groups were then subjected to 30 min global I followed by 2 h of R.
Perfusion technique
Male Wistar rats (246.9 ± 4.7 g) were anesthetized (sodium pentobarbitone, 60 mg/kg, i.p.) and heparinized (500 IU, i.p.). Hearts were excised and rapidly mounted on a Langendorff perfusion apparatus. Retrograde perfusion in a non-recirculating mode was established at a constant perfusion pressure of 70 mmHg and 37°C. KHB gassed with 95% O2 and 5% CO2 (pH 7.4) containing (in mM) NaCl 118.0; KCl 3.0; MgSO4 1.2; NaHCO3 27.2; KH2PO4 1.18; CaCl2 1.76; and glucose 11.1 was used as the perfusion medium. Perfusate was filtered through a 5 μm porosity filter (Millipore). An epicardial electrogram was registered by means of two stainless steel electrodes attached to the apex of the heart and the aortic cannula. Left ventricular pressure was measured by means of a non-elastic water-filled balloon inserted into the left ventricle via the left atrium connected to a pressure transducer (MLP844 Physiological Pressure Transducer; ADInstruments) and adjusted to obtain a left ventricular end-diastolic pressure (LVEDP) of 4–7 mmHg. Left ventricular developed pressure (LVdevP: LV systolic minus LV diastolic pressure), LVEDP, and +dP/dtmax (as an index of contraction) were monitored continuously until 40 min of R when the balloon was deflated, while heart rate (HR; derived from an electrogram) and coronary flow (CF) were recorded until the end of reperfusion. Heart function and arrhythmias were analyzed using PowerLab/8SP Chart 5 software (ADInstruments).
Quantification of arrhythmias
Susceptibility to R-induced ventricular arrhythmias was analyzed from the electrogram recording during the first 10 min of R, as per the Lambeth Conventions (CitationWalker et al., 1988). We focused on measurement of the total number of ventricular premature beats (VPB), as well as the total duration of episodes of ventricular tachycardia (VT), which was defined as a run of four or more consecutive ectopic beats.
Infarct size determination
At the end of 2 h R, the hearts were stained with 1% 2,3,5-triphenyltetrazolium chloride (Sigma, USA) dissolved in 0.1 M phosphate buffer (pH 7.4). The hearts were then stored overnight in 10% neutral formaldehyde solution and cut perpendicularly to the long axis of the ventricle into 1 mm thick slices. The infarct area (IA) and the area at risk (AR) were measured by a computerized planimetry method. The infarct size was normalized to the size of the area at risk (IA/AR).
Statistical evaluation
The data are expressed as mean ± SEM. Student’s t-test was used to compare the difference between the control and treated groups. The Mann–Whitney U-test was used to compare the differences in the number of VPB and duration of VT. Differences were considered significant when p ≤ 0.05.
Results and discussion
Myocardial injury caused by 30 min of I followed by 120 min of R has been observed to produce irreversible cardiac damage (CitationPalmer et al., 2004). Thus, this model can be used to study the effect of drugs on severe irreversible myocardial insult. There were no significant changes in LVdevP (88.7 ± 3.8 mmHg) and HR (286.6 ± 23.9 beats per minute (BPM)) after 15 min of perfusion with HI, compared to values of 97.2 ± 5.0 mmHg and 307.8 ± 14.7 BPM before drug administration, but there was a slight increase in CF from 9.7 ± 0.7 mL/min to 12.7 ± 1.7 mL/min. This might be related to the effect of some components of the extract that may exert vasodilatory effects.
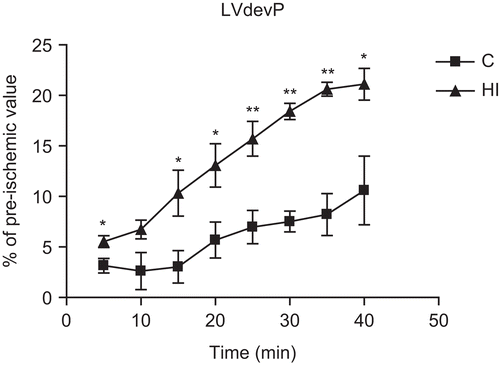
In the post-ischemic period, the recovery of LVdevP was significantly higher at almost all time points except 10 min, as compared with C (). The recovery of +dP/dtmax at 40 min of R was also significantly higher (p < 0.05) and the LVEDP was significantly lower (p < 0.01) at 40 min of R than in C, indicating an improved recovery of contractile function and attenuation of diastolic contracture developed during I/R. The recovery of CF was increased by HI, but the difference was not significant. The total number of VPB and the duration of VT were significantly lower than in C (), indicating that HI might have a protective effect on the pacemaker function and protect the heart against severe arrhythmias after I/R insult. There was also a slight reduction in the size of infarction, although it did not reach the level of significance.
Table 1. Effect of Hemidesmus indicus (HI) on post-ischemic functional recovery, reperfusion arrhythmias, and infarct size in the isolated rat heart.
Figure 1. Effect of Hemidesmus indicus (HI) on post-ischemic recovery of left ventricular developed pressure (LVdevP). C, control non-treated group; HI, HI-treated group. Data are mean ± SEM (n = 7 per group). *p < 0.05, **p < 0.01 vs. C.
Functional deteriorations and severe arrhythmias upon reperfusion are related to a certain extent to an excessive generation of reactive oxygen species during prolonged I/R and their deleterious effects on cardiac cell membranes and function of ion transport systems (CitationRavingerová et al., 1999; CitationKaplán et al., 2003). This has been verified by the efficacy of antioxidants and scavengers in experimental settings of acute I/R (CitationRay et al., 1999; CitationMakazan et al., 2007). Similarly, pretreatment with antioxidants such as melatonin (CitationSzárszoi et al., 2001; CitationVažan et al., 2005) and N-acetylcysteine (CitationMatejíková et al., 2009) prior to I reduced the severity and duration of R-induced ventricular arrhythmias in isolated perfused rat hearts, attenuated calcium overload of the heart (CitationVažan et al., 2003), and improved post-ischemic recovery of contractile function (CitationSzárszoi et al., 2001). Further, attenuation of inflammation has been shown to be beneficial in myocardial injury (CitationWang et al., 2006).
HI possesses an antioxidant effect (CitationMary et al., 2003; CitationRao et al., 2005), which might be due to tannins (CitationHong et al., 1995), some of its main constituents. Further, saponins are shown to have beneficial effects on cardiovascular diseases (CitationMatsuura, 2001). Hence, the ingredients of HI, e.g., tannins and saponins, might contribute to the observed beneficial effects. In addition, HI is also known to have an anti-inflammatory effect Citation(Lakshman et al., 2006). It is, therefore, conceivable that the antioxidant and anti-inflammatory properties of HI may be a reason for the improvement of functional recovery and suppression of R-induced arrhythmias, as well as the tendency toward mitigation of irreversible changes in I/R hearts as demonstrated in our study.
In conclusion, HI was able to protect the heart against post-ischemic myocardial stunning and R-induced arrhythmias in a setting of irreversible I/R injury, and further study with an increased dose of HI and detailed investigation of the mechanism of its action is encouraged.
Acknowledgements
We also acknowledge Rumi Herbals for providing the extract.
Declaration of interest
The study was supported by grant number VEGA SR 2/0173/08, and SAIA is thanked for providing a scholarship to one of the authors (V.K.M.K.).
References
- Anjaria J, Parabia M, Bhatt G, Khamar R (2002). Nature Heals, a Glossary of Selected Indigenous Medicinal Plants of India. Ahmedabad, SRISTI Innovations, pp. 31.
- Anoop A, Jegadeesan M (2003): Biochemical studies on the anti-ulcerogenic potential of Hemidesmus indicus R. Br. Var. J Ethnopharmacol 84: 149–156.
- Bopanna KN, Bhagyalakshmi N, Rathod SP, Balaraman R, Kannan J (1997): Cell culture derived Hemidesmus indicus in the prevention of hypercholesterolemia in normal and hyperlipidemic rats. Cell 29: 105–109.
- Hong CY, Wang CP, Huang SS, Hsu FL (1995): The inhibitory effect of tannins on lipid peroxidation of rat heart mitochondria. J Pharm Pharmacol 47: 138–142.
- Kaplán P, Babušiková E, Lehotský J, Dobrota D (2003): Free radical-induced protein modification and inhibition of Ca 2+ ATPase of cardiac sarcoplasmic reticulum. Mol Cell Biochem 248: 41–47.
- Lakshman K, Shivaprasad HN, Jaiprakash B, Mohan S (2006): Anti-inflammatory and antipyretic activities of Hemidesmus indicus root extract. Afr J Tradit Complement Altern Med 3: 90–94.
- Makazan Z, Saini HK, Dhalla NS (2007): Role of oxidative stress in alterations of mitochondrial function in ischemic-reperfused hearts. Am J Physiol Heart Circ Physiol 292: H1986.
- Mary NK, Achuthan CR, Babu BH, Padikkala J (2003): In vitro antioxidant and antithrombotic activity of Hemidesmus indicus (L) R. Br. J Ethnopharmacol 87: 187–191.
- Matejíková J, Kucharská J, Pintérová M, Pancza D, Ravingerová T (2009): Protection against ischaemia-induced ventricular arrhythmias and myocardial dysfunction conferred by preconditioning in the rat heart: Involvement of mitochondrial K(ATP) channels and reactive oxygen species. Physiol Res 58: 9–19.
- Matsuura H (2001): Saponins in garlic as modifiers of the risk of cardiovascular disease. J Nutr 131: 1000S–1005S.
- Murshed S, Rokeya B, Nahar N, Khan AKA, Mosihuzzaman M, Banerjee A, Maiti S, Ali L (2005): Hypoglycemic and hypolipidemic effect of Hemidesmus indicus root on diabetic model rats. Diabetes Res 39: 15–24.
- Palmer BS, Hadziahmetovic M, Veci T, Angelos MG (2004): Global ischemic duration and reperfusion function in the isolated perfused rat heart. Resuscitation 62: 97–106.
- Prabakan M, Anandan R, Devaki T (2000): Protective effect of Hemidesmus indicus against rifampicin and isoniazid-induced hepatotoxicity in rats. Fitoterapia 71: 55–59.
- Rao GMM, Venkateswararao C, Rawat AKS, Pushpangadan P, Shirwaikar A (2005): Antioxidant and antihepatotoxic activities of Hemidesmus indicus R. Br. Acta Pharm Turc 47: 107–113.
- Ravingerová T, Slezák J, Tribulová N, Džurba A, Uhrík B, Ziegelhöffer A (1999): Free oxygen radicals contribute to high incidence of reperfusion-induced arrhythmias in isolated rat heart. Life Sci 65: 1927–1930.
- Ray PS, Maulik G, Cordis GA, Bertelli AAE, Bertelli A, Das DK (1999): The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Rad Biol Med 27: 160–169.
- Suchalatha S, Devi CSS (2004): Effect of Arogh – a polyherbal formulation – on the marker enzymes in isoproterenol induced myocardial injury. Indian J Clin Biochem 19: 184–189.
- Szárszoi O, Asemu G, Vanecek J, Ošt’ádal B, Kolár F (2001): Effects of melatonin on ischemia and reperfusion injury of the rat heart. Cardiovasc Drugs Ther 15: 251–257.
- Važan R, Pancza D, Béder I, Styk J (2005): Ischemia reperfusion injury antiarrhythmic effect of melatonin associated with reduced recovering of contractility. Gen Physiol Biophys 24: 355–359.
- Važan R, Styk J, Béder I, Pancza D (2003): Effect of melatonin on the isolated heart in the standard perfusion conditions and in the conditions of calcium paradox. Gen Physiol Biophys 22: 41–50.
- Walker MJA, Curtis MJ, Hearse DJ, Campbell RWF, Janse MJ, Yellon DM, Cobbe SM, Coker SJ, Harness JB, Harron DWG (1988): The Lambeth Conventions: Guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc Res 22: 447–455.
- Wang M, Tsai BM, Reiger KM, Brown JW, Meldrum DR (2006): 17-β-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol 40: 205–212.