Abstract
Clematis brachiata Thunb. (Ranunculaceae) is used as a folk remedy for the treatment of pain, fever and inflammatory ailments. Aqueous extract of Clematis brachiata leaf was screened for its phytochemical constituents. The anti-inflammatory investigations were carried out using carrageenan and histamine-induced edema models; acetic acid writhing, formalin-induced pain and tail immersion models were used to evaluate antinociceptive activity while a Brewer’s yeast-induced hyperthermia model was employed for the antipyretic experiment. Phytochemical screening of the extract revealed the presence of tannins, saponins, flavonoids and cardiac glycosides. The extract at 100, 200 and 400 mg/kg body weight significantly (P<0.05) reduced the edema paw volumes induced by carrageenan and histamine with the 400 mg/kg body weight extract being the most potent. On the antinociceptive front, while the extract reduced the writhing caused by acetic acid and the number of licks induced by formalin in a dose dependent manner, the increase in the reaction time by the extract in the tail immersion model was not dose-dependent. Again, there was significant (P<0.05) lowering of the Brewer’s yeast-provoked elevated body temperature. The results suggest that the aqueous extract of Clematis brachiata leaves can be employed in the management of inflammation, pain and fever. These activities may be due in part to the flavonoid content of the extract.
Introduction
Anti-inflammatory, analgesic and antipyretic drugs have not been used successfully in all cases due to adverse side effects such as gastric lesions caused by non-steroidal anti-inflammatory drugs (NSAIDS). Therefore, new drugs lacking these side effects are searched for all over the world. During this process, the investigation of the efficacy of plant-based drugs used in traditional medicines for the treatment of pain, fever and inflammatory ailments have received attention because they are cheap and have few side effects (CitationKumara, 2001).
Clematis brachiata Thunb. (Ranunculaceae), known as traveler’s joy (English), Ityolo (Xhosa) and Umdlonzo (Zulu) is widely distributed in South Africa, Swaziland, Namibia and Botswana. It is a thin, twining, woody deciduous climber that grows up to 5 m in length. It bears masses of small, sweetly scented, creamy white flowers in the late summer and autumn. It is claimed to possess wonderful pain relieving properties and was useful to travelers when they traveled long distance by foot (CitationRoberts, 1990). The leaves are used by the Xhosas, Zulus, and Tswanas to ease headaches, coughs, chest ailments and abdominal upsets (CitationRoberts, 1990). Hot decoctions of the leaves, stems and roots are also used separately to relieve cold, malaria, sinus infections and asthma (CitationChhabra et al., 1991; CitationKoch et al., 2005; CitationPendota et al., 2008). In addition, an infusion of the leaves and stem bark is used for treating schistomiasis in South Africa (Spang et al., Citation2000).
The folkloric claim of Clematis brachiata leaves to relieve pain, fever, and inflammation has no supporting scientific evidence in literature. Therefore, the present study evaluated the anti-inflammatory, antinociception, and antipyretic properties of the aqueous extract of C. brachiata leaves in male Wistar rats.
Materials and methods
Plant collection
Clematis brachiata leaves were collected in April, 2008 from a natural population growing within the premises of University of Fort Hare, Alice, South Africa. The plant was identified by Prof. D.S. Grierson of the Department of Botany, University of Fort Hare, and a voucher specimen (M. Mostafa med. 2008/1) was deposited at the Giffen Herbarium of the University.
Chemicals
Carrageenan, acetic acid, formalin, histamine, Brewer’s yeast and indomethacin were products of Sigma-Aldrich, Steinheim, Germany. All other chemicals used were of analytical grade and were supplied by Merck, Bellville, South Africa.
Preparation of extract
The leaves were oven-dried at 40°C for 48 h and thereafter pulverized. The powder (200 g) was boiled in 1L of distilled water for 20 min and allowed to cool for 2 h. The extract was then filtered with a Buchner funnel and Whatman No. 1 filter paper (Maidstone, UK). The filtrate was later freeze-dried using a Savant Refrigerated Vapor Trap, (RVT4104, California, USA) to give a yield of 45 g. This was reconstituted in distilled water to give the required doses used for each experiment. The doses used for the various studies were in line with what the traditional healers use for treatments.
Experimental animals
One hundred and eighty male albino rats (Rattus norvegicus) of Wistar strain weighing between 180-200 g were obtained from the Experimental Animal House of the Agriculture and Rural Development Research Institute, University of Fort Hare, Alice. The rats were completely randomized into six groups of 30 for each part of the experiment. The animals were kept in rat cages and fed on Balanced Trusty Chunks (Pioneer Foods, Huguenot, South Africa), and clean water ad libitum. This study was carried out following approval from the ethical committee on the use and care of animals of the University of Fort Hare, South Africa.
Phytochemical screening
Phytochemical screening of the extract was carried out for alkaloids, saponins, tannins, flavonoids, anthraquinones, steroids, phenols and glycosides using the standard procedures described by CitationHarborne (1973), CitationTrease & Evans, (1989), and CitationSofowora (1993).
Determination of total flavonoids
Total flavonoid of the extracts was estimated using the method described by CitationOrdonez et al., (2006). To 0.5 mL of the extract, 0.5 ml of 2% AlCl3 in ethanol solution was added. The absorbance was read at 420 nm with a spectrophotometer (Beckman Coulter DU 700, Fullerton, California, USA) after being allowed to stand at room temperature for 1 h. Total flavonoid content was calculated and expressed as mg of quercetin equivalent per g using the equation obtained from a standard quercetin calibration curve: y = 43.862 × - 0.1757, R2 = 0.9931.
Pharmacological activities
Anti-inflammatory activity
Carrageenan-induced edema test
The method of CitationLanhers et al. (1991) was adopted for the carrageenan-induced edema test in rats. Briefly, edema was induced by injecting 0.05 mL of 1% carrageenan into the sub-plantar region of the right hind paw of each rat. Five groups (six animals per group) were used in this study. Groups A, B and C were orally administered with the extract at 100, 200 and 400 mg/kg body weight respectively while groups D and E received orally 0.5 mL of distilled water and same volume containing 10 mg/kg body weight of indomethacin respectively, 30 min before the carrageenan injection. The paw volume was measured with a micrometer screw gauge (SMC-20326, Sterling Manufacturing Company, Ambala Cantt, India) at 0, 0.5, 1, 2, 4, 6 and 24 h after the administration of the reference drug and the extract. The percentage inhibition of inflammation by the extract was calculated using the expression:
where Vc was the average degree of inflammation in the control group and Vt was the average degree of inflammation in the test groups.
Histamine-induced rat paw edema
Paw edema was produced by sub-plantar administration of 0.1% freshly prepared solution of histamine into the right hind paw of the rats according to the method described by CitationPerianayagam et al. (2006). Rats (six per group) were completely randomized into five groups (A-E) and pretreated as follows: group A received orally 0.5 mL of distilled water and served as the negative control, groups B, C and D were orally administered with 100, 200 and 400 mg/kg body weight of the extract, respectively, while group E received orally 10 mg/kg body weight of indomethacin. The right hind paw volume was measured with a micrometer screw gauge at 0, 1, 2, 4 and 6 h after the administration of the reference drug and the extract. The percentage inhibition of the inflammatory activity was calculated as earlier described for the carrageenan-induced edema test.
Analgesic activity
Acetic acid writhing reflex test
The acetic acid-induced writhing was performed according to the procedure described by CitationGaertner et al. (1999). Rats (six per group) were injected intraperitoneally with 0.6% (v/v) acetic acid at 10 mL/kg body weight. The extract (100, 200, and 400 mg/kg body weight), indomethacin (10 mg/kg body weight) and distilled water (0.5 mL/rat) were administered orally, 30 min prior to the treatment with acetic acid. The writhings (abnormal constrictions and hind limb stretchings) induced by the acetic acid were counted for 30 min after a latency period of 5 min. The percentage analgesic activity was calculated as follows:
where A was the average number of stretchings produced by the distilled water control animals and B was the average number of stretchings in the extract/drug treated animals.
Formalin-induced pain test
The procedure described by CitationSantos et al. (1994) was adopted in the formalin-induced pain experiment. Briefly, pain was induced by injecting 0.05 mL of 2.5% formalin (40% formaldehyde) in distilled water in the sub-plantar region of the right hind paw of the animals. Rats (six per group) were orally administered with the extract (100, 200, 400 mg/kg body weight), indomethacin (10 mg/kg body weight) and water (0.5 mL/rat), 30 min prior to injecting formalin. These animals were thereafter placed individually in a transparent cage of dimension 48.5 cm × 33.5 cm × 22.5 cm. The amount of time spent licking the injected paw was indicative of pain (CitationHunskaar & Hole, 1987). The number of licks from 0-5 min (first phase) and 15-30 min (second phase) after formalin injection were counted. The percentage inhibition of licking was calculated using the expression:
where Lc was the average number of licks of the control group and Lt was the average number of licks of the extract/drug treated groups.
Tail immersion test
This test was conducted according to the method described by CitationAydin et al. (1999). Rats (six per group) were used in this experiment. The extreme 3 cm of the rat tail was immersed in a water bath maintained at a temperature of 55 ± 0.5°C. Within a few minutes, the rat reacted by withdrawing the tail. The reaction time was recorded with a stop watch. Each animal served as its own control and two readings were obtained for the control at 0 and 10 min intervals. The average of the two values was regarded as the initial reaction time (Tb). The animals were orally administered with the extract (100, 200, 400 mg/kg body weight), indomethacin (10 mg/kg body weight) and distilled water (0.5 mL/rat). The reaction time (Ta) for the extract and drug treated groups was taken at 0.5, 1, 2 and 4 h after a latency period of 30 min following the administration of the extract and reference drug (CitationVogel & Vogel, 1997).
Antipyretic test
Fever was induced by Brewer’s yeast following the method described by CitationBrune and Alpermann (1983). Five groups of six rats each were used. An initial rectum temperature was recorded by inserting a clinical thermometer (Panamedic, Cheonan Choongnam, Korea), 2 cm deep into the rectum of the animals. This was recorded after 30 min and the average was calculated. The rats were given fevers by the injection of 20 mg/kg body weight of Brewer’s yeast suspension administered subcutaneously into the nurque region of the animal’s neck. Following the injection, the site was massaged in order to spread the suspension beneath the skin. Room temperature was kept at 31.5°C. The animals were starved of food for 18 h (but with adequate water) before the commencement of the experiment. The rectal temperature was recorded 18 h post-injection and rats with body temperature greater than 37.2°C were used for the experiment. The rectal temperature was measured from 0.5-6 h post-dosing.
Statistical analysis
Data obtained were subjected to one way analysis of variance (ANOVA) and means were separated by Duncan Multiple Range Test. Significant levels were tested at P <0.05.
Results
Phytochemical analysis of the aqueous extract of C. brachiata leaves revealed the presence of tannins, saponins, flavonoids and cardiac glycosides. The anti-inflammatory effects of the aqueous extract of Clematis brachiata leaves were assessed using carrageenan and histamine-induced edema models. In the carrageenan-induced edema test, a maximum edema paw volume of 1.76 ± 0.06 mm was observed in the control rats, 4 h after the carrageenan injection (). Rats pre-treated with the extract at 200 and 400 mg/kg body weight significantly decreased (p<0.05) the carrageenan-induced edema paw volume, 30 min post dosing, whereas the 100 mg/kg body weight manifested 2 h after treatment with the phlogistic agent. The trend of reduction in the paw volume by the 400 mg/kg body weight of the extract compared favorably with the indomethacin treated animals at 6 and 24 h. Although, the extracts and indomethacin treatment reduced the swellings, they were still significantly visible after 24 h.
Table 1. Effect of aqueous extract of Clematis brachiata leaves on carrageenan-induced edema paw volume in male Wistar rats; n = 6, × ± SD.
In the histamine-induced edema model, the injection of the irritant histamine caused localized edema after 1 h (). The size of the right hind paw volume increased to a maximum of 1.18 ± 0.04 mm between 2 and 4 h after the injection of histamine. Rats pre-treated with C. brachiata extract significantly decreased the histamine-induced right hind paw volume with that of 100 and 200 mg/kg body weight comparing well with each other. The inhibition of edema formation by the 400 mg/kg body weight of the extract after 6 h was similar to that produced by the indomethacin ().
Table 2. Effect of aqueous extract of Clematis brachiata leaves on indomethacin on histamine-induced edema in male Wistar rats; n = 6, × ± SD.
The extract also showed significant analgesic activity in the acetic acid writhing model (). There was a dose-dependent decrease in the number of writhings by the extract within the 30 min observation period. The computed percentage inhibition of the organic acid induced abdominal constrictions was also dose-dependently increased, with that of 200 and 400 mg/kg body weight treated animals producing higher inhibitions than the indomethacin treated animals.
Table 3. Effect of aqueous extract of Clematis brachiata leaves on writhing induced by acetic acid in male Wistar rats; n = 6, × ± SD.
The extract also exhibited analgesic effect on both the first (0-5 min) and the second (15-30 min) phases of the formalin-induced pain model (). The extract dose-dependently decreased the number of licks during the neurogenic (0-5 min) and inflammatory pain (15-30 min) phases. The inhibition also increased in a dose-related manner. The 400 mg/kg body weight of the extract produced a better analgesic effect than the reference drug, although, the doses are not the same.
Table 4. Effect of aqueous extract of Clematis brachiata leaves on formalin-induced pain in male Wistar rats; n = 6, × ± SD.
Administration of Clematis brachiata leaf extract and indomethacin significantly increased the reaction time of the rats to the warm water-induced pain (). While the increase was dose related in the indomethacin and 400 mg/kg body weight extract-treated animals, those of 100 and 200 mg/kg body weight of the extract were not dose-dependent.
Table 5. Effect of aqueous extract of Clematis brachiata leaves on tail immersion test in Wistar rats; n = 6, × ± SD.
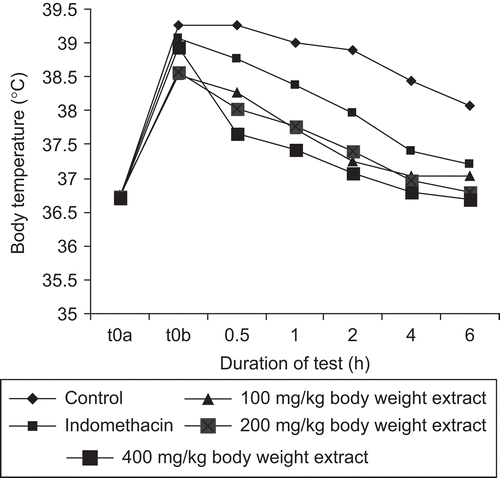
While the distilled water-treated control animals remained hyperpyretic throughout the experimental period, the extract significantly reduced the Brewer’s yeast-provoked body temperature. The reduction in the body temperature was immediate for the 400 mg/kg body weight and indomethacin-treated animals, whereas this was delayed until 1 h after administration in the 100 and 200 mg/kg body weight extract-treated groups. The lowering effect of the body temperature by the 400 mg/kg body weight of the extract was more pronounced than in the indomethacin treated animals ().
Discussion
Acute inflammation involves the synthesis or release of mediators at the injured site. These substances, which include prostaglandins (especially the E series), histamine, bradykinins, leukotrienes, and serotonin, apart from initiating inflammation also cause pain and fever. Therefore, inhibition of these mediators from reaching the injured site or from bringing about their pharmacological effect will normally ameliorate inflammation, pain and fever (CitationWu, 2003; CitationSawadogo et al., 2006).
Carrageenan-induced paw edema, an in vivo model of inflammation, has been frequently used to assess the anti-edematous effect of natural products (CitationMani Senthil Kumar et al., 2008). It has also been reported that various mediators are released by carrageenan in the rat paw. The initial phase may be due to histamine, while the second phase is attributed to prostaglandins (CitationMossa et al., 1995). Development of edema induced by carrageenan is commonly correlated with the early exudative stage of inflammation, one of the important processes of inflammatory pathology (CitationOzaki, 1990). The significantly decreased edema paw volume of the animals as well as the increase in inhibition of inflammation by the extract at all the doses investigated suggests anti-inflammatory potential. The 400 mg/kg dose of the extract was the most potent and produced an anti-inflammatory effect that was similar to indomethacin, a well known prostaglandin inhibitor. Therefore, the anti-inflammatory property of the extract could be due to its ability to inhibit the cyclooxygenase pathway, considering that the mechanism involved in the genesis of carrageenan-induced edema could cause the release of prostaglandins and kinins, as well as other substances (CitationGarcialeme et al., 1973).
Histamine is an important inflammation mediator as well as a potent vasodilator which increases vascular permeability (CitationMossa et al., 1995; CitationCuman et al., 2001; CitationLinardi et al., 2002). The suppression of histamine-induced edema by Clematis brachiata extract may be attributed to anti-inflammatory actions. This could be due to the ability of the extract to inhibit the synthesis, release or action of inflammatory mediators, such histamine, serotonin and prostaglandins (CitationAdedapo et al., 2008). Flavonoids have been reported to be famous for their anti-inflammatory activity due to the influence on the production of prostaglandins (CitationMonsef et al., 2004; CitationMohajer et al., 2005). Therefore, the inflammatory activity of Clematis brachiata leaf extract in this study may be associated with its flavonoid content.
The acetic acid-induced abdominal writhing is a visceral pain model in which the processor releases arachidonic acid via cyclooxygenase, and prostaglandin biosynthesis plays a role in the nociceptive mechanism (CitationFranzotti et al., 2002). Acetic acid induces pain by increasing fluids of PGE2 and PGF2α (CitationDeraedt et al., 1980) at the peritoneal receptors (CitationBentley et al., 1983). The organic acid has also been postulated to act indirectly by inducing the release of endogenous mediators, which stimulates the nociceptive neurons that are sensitive to NSAIDs and narcotics (CitationAdzu et al., 2003). The antagonized acetic acid-induced writhing by the extract at all the doses suggests antinociceptive effect which might have resulted from the inhibition of the synthesis of arachidonic acid metabolites. The inhibitory effects of the 200 and 400 mg/kg body weight of the extract on the abdominal constrictions which were higher than those produced by indomethacin suggests that the extract at these doses was more potent than the reference drug as antinociceptive agent, notwithstanding the disparity in the dosages.
The formalin test that can be used to assess the effect of plant extract on chronic pain is a better model than the acetic acid and tail immersion, which are only for acute pain (CitationCowan, 1990). The formalin model normally postulates the site and the mechanism of action of the analgesic (CitationChau, 1989). This biphasic model is represented by neurogenic (0-5 min) and inflammatory pain (15-30 min), respectively (CitationHunskaar & Hole, 1987). The responses consist of licking and lifting of the injected paw, flinching and also protection of the paw from full pressure when walking or resting (CitationPorro & Cavazzuti, 1993). The significant inhibitory effect on both phases of nociceptive response by the extract in this study suggests inhibition of inflammatory mediators, notably prostaglandin synthesis as well as the blockade of its receptor. Drugs that act primarily on the central nervous system such as narcotics inhibit both phases equally while peripherally acting agents such as steroids and NSAIDS suppress mainly the late phase (CitationAdzu et al., 2003). The suppression of neurogenic and inflammatory pains by the extract might imply that it contains active analgesic principle that may be acting both centrally and peripherally. This is an indication that the extract can be used to manage acute as well as chronic pain.
The tail immersion model is used to determine acute pain (CitationFranzotti et al., 2002; CitationAsongalem et al., 2004). Tail flick response is predominantly considered to be selective for centrally acting analgesics, while the peripherally acting is known to be inactive on this kind of painful stimulus (CitationSrinivasan et al., 2003). In this study, the significantly increased reaction times of the extract-treated animals to the pain induced by the warm water suggest analgesic activity of the plant. Similarly, the sensitivity produced by the experimental animals to the tail flick response could imply that the extract is a centrally acting analgesic. This further corroborates the findings in the formalin test. The non-steroidal anti-inflammatory and analgesic effect of this extract may be attributed to the overall effect of the plant constituents or the compounds having action similar to NSAIDs (CitationAdedapo et al., 2008). The antinociceptive effect of C. brachiata may be related to the reduction in the influx of calcium ions at the terminal of the axon of the afferent nerve resulting in a corresponding decrease in the activity of adenylyl cyclase. The reduction in the activity of the enzyme will lead to decreased levels of cyclic AMP, and subsequently efflux of potassium ion. The resultant hyperpolarization of the nerves will confer antinociception (CitationYaksh, 1999).
Fever may occur as a result of infection or one of the sequelae of tissue damage, inflammation, graft rejection, or other disease states (CitationRao et al., 2002). Regulation of body temperature requires a delicate balance between the production and loss of heat, and the hypothalamus regulates the set point at which body temperature is maintained. Therefore, the significant reduction in the brewer’s yeast provoke elevated body temperature in the animals suggests antipyretic potential of the plant extract. Studies have shown that alkaloids have the ability to inhibit the synthesis of prostaglandin E2 (CitationBackhouse et al., 1994), eventually reducing elevated body temperature in animals. Similarly, flavonoids have been implicated as an antipyretic agent by suppressing TNF-α (CitationChang et al., 2007). The antipyretic properties of Clematis brachiata could possibly be associated with the flavonoids since alkaloid was not detected in the plant extract.
The results of the present study have shown that the aqueous extract of Clematis brachiata leaves could be explored in the management of pain, inflammation and fever. The potential of the extract of Clematis brachiata leaves as anti-inflammatory, antinociceptive and antipyretic agents may be due in part to the phytoconstituents, especially the flavonoid content which is about 10.03 mg/g.
Declaration of interest
The authors acknowledge support from the Govan Mbeki Research and Development Center, University of Fort Hare, South Africa, and the Bangladesh Council of Scientific and Industrial Research (BCSIR), Bangladesh. The authors report no conflict of interest.
References
- Adedapo AA, Sofidiya MO, Maphosa V, Moyo B, Masika PJ, Afolayan AJ (2008): Anti-inflammatory and analgesic activities of the aqueous extract of Cusssonia paniculata stem bark. Rec Nat Prod 2: 46–53.
- Adzu B, Amos S, Kapu SD, Gamaniel KS (2003): Anti-inflammatory and anti-nociceptive effects of Sphaeranthus senegalensis. J Ethnopharmacol 84: 169–173.
- Asongalem EA, Foyet HS, Ngogang J, Folefoc GN, Dimo T, Kamtchouing P (2004): Analgesic and anti-inflammatory activities of Erigeron floribundus. J Ethnopharmacol 91: 301–308.
- Aydin S, Demir T, Ozturk Y, Base KHC (1999): Analgesic activity of Nepeta italica L. Phytother Res 13: 20–23.
- Backhouse N, Delporte C, Givemau M, Cassels BK, Valenzuela A, Speisky H (1994): Anti-inflammatory and antipyretic effects of Boldine. Inflam Res 42: 114–117.
- Bentley GA, Newton SH, Starr J (1983): Studies on the anti-nociceptive action of α-agonist drugs and their interaction with opioid mechanisms. Br J Pharmacol 79: 125–134.
- Brune K, Alpermann H (1983): Non-acidic inhibition of prostaglandin production, carrageenan edema and yeast fever. Agent Actions 13: 360–363.
- Chang CP, Huang WT, Cheng BC, Hsu CC, Lin MT (2007): The flavonoid baicalin protects against cerebrovascular dysfunction and brain inflammation in experimental heatstroke. Neuropharmacology 52: 1024–1033.
- Chau TT (1989): Analgesic Testing in Animal Models, Pharmacological Methods in the Control of Inflammation, New York, Liss, p. 195.
- Chhabra SC, Mahunnah RLA, Mshiu EN (1991): Plants used in traditional medicine in Eastern Tanzania. V. Angiosperms (Passifloraceae to Sapindaceae). J Ethnopharmacol 33: 43–157.
- Cowan A (1990): Recent approaches in the testing of analgesics in animals. Modern methods in pharmacology. Alder MW and Cowan A (eds.). Testing and Evaluation of Drugs Abuse, Vol. 6. New York: Wiley Liss, pp. 33–42.
- Cuman RKN, Bersani-Amadio CA, Fortes ZB (2001): Influence of type 2 diabetes on the inflammatory response in rat. Inflammation Res 50: 460–465.
- Deraedt R, Jougney S, Delevalence F, Falhout M (1980): Release of prostaglandin E and F in an analgesic reaction and its inhibition. Eur J Pharmacol 51: 17–24.
- Franzotti EM, Santos CVF, Rodrigues HMSL, Mourao RHV, Andrade MR, Antoniolli AR (2002): Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. J Ethnopharmacol 72: 273–278.
- Gaertner M, Muller L, Roos JF, Cani G, Santos ARS, Niero R, Calixto JF, Yunes RA, Delle Monache F, Cechinel-Fehho V (1999): Analgesic triterpenes from Sebastiania schottianan roots. Phytomedicine 6: 41–44.
- Garcialeme J, Nakamura L, Leite MP, Rochae SM (1973): Pharmacological analysis of the acute inflammatory process induced in rat’s paw by local injection of carrgeenan and by heating. Br J Pharmacol 48: 88–96.
- Harborne JB (1973): Phytochemical Methods. London, Chapman and Hall, pp. 49–188.
- Hunskaar S, Hole K (1987): The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 30: 103–114.
- Koch A, Tamez P, Pezzuto J, Soejarto D (2005): Evaluation of plants for antimalarial treatment by the Masai of Kenya. J Ethnopharmacol 101: 95–99.
- Kumara NKHMR (2001). Identification of strategies to improve research on medicinal plants used in Sri Lanka. In: WHO Symposium. University of Ruhuna, Galle, Sri Lanka. pp. 12–14.
- Lanhers MC, Fleurentin J, Dorfman P, Motrier F, Pelt JM (1991): Analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta. Planta Med 57: 225–231.
- Linardi A, Costa SKP, De Silva GR, Antune E (2002): Involvement of kinins, mast cells and sensory neurones in the plasma exudation and paw edema induced by Staphylococcal entrotoxin B in the mouse. Eur J Pharmacol 399: 235–242.
- Mani Senthil Kumar KT, Goran B, Roy DK, Zothanpuia Samantha, SK, Pal M, Biswas P, Roy A, Adhikari D, Karmakar S, Sen T (2008): Anti-inflammatory activity of Acanthus ilicifolius. J Ethnopharmacol 120: 7–12.
- Mohajer M, Sarkhail P, Hajarolasvadi N, Zamani MJ, Khorasani R, Shafiee A, Amin G, Abdollahi M (2005): Antiinflammatory and analgesic effects of Phlomis lanceolata Boiss. and Hohen. extracts and examination of their components. Int J Pharmacol 2: 50–54.
- Monsef RZ, Ghobadi A, Iranshahi M (2004): Antinociceptive effect of Peganum harmala L. alkaloid on mouse formalin test. J Pharm Pharmaceut Sci 7: 70–75.
- Mossa JS, Rafatullah S, Gala AM, Al-Yahya MA (1995): Pharmacological studies of Rhus retinorrhaea. Int J Pharmacog 33: 242–246.
- Ordonez AAL, Gomez JD, Vattuone MA, Isla MI (2006): Antioxidant activity of Sechium edule (Jacq.) Swart extracts. Food Chem 97: 431–437.
- Ozaki Y (1990): Anti-inflammatory effects of Curcuma xanthorrhiza Roxb., and its active principle. Chem Pharm Bull 38: 1045–1048.
- Pendota SC, Grierson DS, Afolayan AJ (2008): An ethnobotanical study of plants used for the treatment of eye-infections in the Eastern Cape, South Africa. Pak J Bio Sci 11: 2051–2053.
- Perianayagam JB, Sharma SK, Pillai KK (2006): Anti-inflammatory activity of Trichodesma indicum root extract in experimental animals. J Ethnopharmacol 104: 410–414.
- Porro CA, Cavazzuti M (1993): Spatial and temporal aspects of spinal cord and brainstem activation in the formalin pain model. Prog Neurobiol 41: 565–607.
- Rao RB, Anupama K, Anand Swaroop Murugesan T, Pal M, Mondal SC (2002): Evaluation of anti-pyretic potential of Ficus racemosa bark. Phytomed 9: 731–733.
- Roberts M (1990): Indigenous Healing Plants. Cape Town, Creda Press, pp. 1–285.
- Santos ARS, Filho VC, Niero R, Viana AM, Morfno FN, Campos MM, Yunes RA, Calixto JB (1994): Analgesic effects of Callus culture extracts from selected species of Phyllanthus in mice. J Pharm Pharmacol 46: 755–759.
- Sawadogo WR, Boly R, Lompo M, Some N (2006): Anti-inflammatory, analgesic and antipyretic activities of Dicliptera verticillata. Intl J Pharmacol 2: 435–438.
- Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Raviprakash V, Kumar D (2003): Anti-nociceptive and antipyretic activities of Pongamia pinnata leaves. Phytother Res 17: 259–264.
- Sofowora A (1993): Medicinal Plants and Traditional Medicine in Africa. Spectrum, Ibadan, Nigeria. p. 289.
- Spang SG, Van Staden J, Jäger AK 2000: Efficiency of traditionally used South African plants against schistosomiasis. J Ethnopharmacol 73: 209–214.
- Trease GE, Evans WC (1989): A Textbook of Pharmacognosy, thirteenth edition. London, Bailliere-Tindall, pp. 582–591.
- Vogel GH, Vogel WH (1997): Analgesic, anti-inflammatory and antipyretic activity. In: Drug Discovery and Evaluation, Pharmacological Assays, New York. p. 360–418.
- Wu KK (2003): Aspirin and other cyclooxygenase inhibitors: new therapeutic insights. Seminar Vascular Med 3: 107–112.
- Yaksh TL (1999): Spinal systems and pain processing:Development of novel analgesics drugs with mechanistically defined models. Trend Pharmacol Sci 20: 329–336.