Abstract
In the present study, the ethanol extract of stem bark of Polyalthia longifolia Benth. and Hook (Annonaceae) was screened for its in vitro and in vivo antitumor activity. In vitro cytotoxicity of P. longifolia extract was assessed in murine cancer cells and in human cancer cells by Trypan blue exclusion assay and MTT assay, respectively. P. longifolia extract showed concentration-dependent cytotoxicity in Ehrlich’s ascites carcinoma (EAC) and Dalton’s ascites lymphoma (DLA) cells with IC50 values of 45.77 and 52.52 µg/mL, respectively. In the MTT assay, the IC50 values of P. longifolia extract against HeLa and MCF-7 cells were 25.24 and 50.49 µg/mL, respectively. In vivo antitumor activity against Ehrlich’s ascites tumor and Dalton’s solid tumor models was assessed by administering 50 and 100 mg/kg of P. longifolia extract, i.p., for 7 consecutive days. P. longifolia extract, at a dose of 100 mg/kg, significantly enhanced mean survival time (MST) and marginally improved hematological parameters when compared to EAC control mice. And the same dose significantly reduced the tumor volume as compared to control DLA inoculated mice. Positive control, cisplatin (3.5 mg/kg, i.p., single dose), significantly enhanced MST and improved hematological parameters when compared to EAC and significantly reduced the tumor volume when compared to DLA control. In vitro antioxidant potential of P. longifolia extract was also determined owing to the role of reactive oxygen species in tumor initiation and progression. P. longifolia extract scavenged DPPH radicals, reduced ferric ions and inhibited lipid peroxidation with IC50 values of 18.14, 155.41 and 73.33 µg/mL, respectively.
Introduction
The search for a selective and less toxic molecule for cancer treatment is an ongoing process. Plants have played an important role as a source of effective anticancer agents and about 60% of the currently available anticancer drugs are derived from plant sources (CitationNewman et al., 2003). The global trend is also towards natural bioactive substances due to their low toxicity and cost. The exploration of medicinal plants for their therapeutic efficacy still holds the hope for the treatment and prevention of cancer.
Polyalthia longifolia Benth. and Hook (Annonaceae) (PL) is a commonly planted tree useful for its effectiveness in alleviating noise pollution. In traditional medicine, the bark is used to treat skin diseases, fever, diabetes, hypertension, and helmenthiasis (CitationRastogi, 1997). Earlier work on Polyalthia longifolia reports the isolation of two clerodane diterpenoids with antifeedant properties from the acetone extract of leaves (CitationPhadnis et al., 1988). The cytotoxic activity of methanol extract of PL stem parts has been reported against KB and P-388 cells (CitationWu & Duh, 1990). Diterpenoids isolated from the hexane extract of P. longifolia seeds demonstrated significant antibacterial and antifungal activities (CitationMurthy et al., 2005). CitationMalairajan et al. (2006) evaluated the analgesic activity of P. longifolia leaf ethanol extract. The anticancer potential of leaf extract of P. longifolia has also been tested in various cancer cell lines and the mechanism of apoptosis induction has also been reported (CitationVerma et al., 2008). But so far the in vivo antitumor and antioxidant activity of the stem bark of PL has not been reported. Hence, the present study focuses the in vitro antioxidant potency and in vitro and in vivo antitumor activity of ethanol extract of Polyalthia longifolia stem bark.
Materials and methods
Cell lines
Dalton’s lymphoma ascites (DLA) and Ehrlich’s ascites carcinoma (EAC) originally obtained from Amala Cancer Research Center, Thrissur, India, were maintained and propagated as ascites tumor in Swiss albino mice by serial intra-peritoneal transplantation at the Central Animal Research Facility, Manipal University, Manipal, India. MCF-7 (human breast adenocarcinoma) and HeLa (human cervical tumor cells) cells procured from the National Center for Cell Science, Pune, India were sub-cultured every two to three days and maintained in 25 cm2 tissue culture flasks (Tarsons Products, Kolkata, India) containing MEM medium supplemented with 10% fetal bovine serum (FBS) and 50 µg/mL gentamicin sulfate at 37°C in a CO2 incubator (NuAire, Plymouth, USA) in an atmosphere of humidified 5% CO2 in 95% air.
Animals
Eight to ten week old Swiss albino mice weighing between 25 and 30 g were selected from an inbred colony maintained under the controlled conditions of temperature (23° ± 2°C), humidity (50% ± 5%) and light (14 h and 10 h of light and dark, respectively). The animals were provided with sterile food and water ad libitum. Four animals were housed in each polypropylene cage containing paddy husk as bedding. Animal care and handling was done according to the guidelines issued by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Plant material and extraction
The fresh stem bark of Polyalthia longifolia was collected in and around Mysore, India, in the month of December and was authenticated by Dr. M. N. Naganandhini, Department of Pharmacognosy, J.S.S. College of Pharmacy, Mysore, Karnataka. Fresh bark of Polyalthia longifolia was shade dried at room temperature and powdered. The bark powder (100 g) was extracted with 95% ethanol in a Soxhlet extractor exhaustively. The extract was then cooled, filtered and concentrated in a rotary flash evaporator. The residue was first dried over a water bath and then in a desiccator over fused calcium chloride. The percentage yield of ethanol extract was found to be 16.8% w/w.
Short-term cytotoxicity studies in DLA and EAC cells
Short-term in vitro cytotoxicity of P. longifolia extract was assessed by the Trypan blue exclusion method (CitationSheeja et al., 1997). In brief, Dalton’s lymphoma ascites and Ehrlich ascites carcinoma (DLA/EAC) cells aspirated from the mice’s peritoneal cavities were washed with PBS two to three times and one million cells were incubated with different concentration of P. longifolia extract in 1 mL of PBS for 3 h at 37°C in sterile test tubes. After incubation, 100 µL of Trypan blue dye (0.4% in PBS) was added and the total number of dead (stained) and viable (unstained) cells were counted using a hemocytometer, and percentage cytotoxicity was calculated using the formula:
where Tdead is the number of dead cells in the treated group, Cdead is the number of dead cells in the control group and Ttot is the total number of dead and live cells in the treated group.
MTT assay in cultured human cancer cells
The effect of P. longifolia extract on growth of cancer cells (MCF-7 and HeLa) was assessed by MTT assay (CitationMossman, 1983). In brief, exponentially growing cells (1 × 104 cells/well) were plated in 96-well plates and allowed to adhere for 24 h prior to extract addition. The extract was dissolved in 0.1% DMSO then diluted with the medium and filtered using 0.22 µ syringe filters. The cells were then exposed to different concentrations of extract (5–200 µg/mL) for 48 h. The cells in the control wells received medium containing the same volume of DMSO (0.1%). After the incubation, 100 µL of MTT reagent (1 mg/mL in PBS) was added and cells were incubated for an additional 4 h. The formazan produced by the viable cells was solubilized by addition of 100 µL DMSO. The suspension was placed on a micro-vibrator for 5 min and absorbance was recorded at 540 nm by the ELISA reader (BIOTEK-ELx800). The experiment was performed in triplicate. Doxorubicin was used as positive control. The percentage of growth inhibition was calculated with respect to vehicle control using the formula
Acute toxicity
Median lethal dose for P. longifolia extract was determined by following the standard Organization of Economic Co-operation and Development Citation(OECD) guidelines (2001). In brief, Swiss albino mice deprived of food for 18 h, were administered various doses of P. longifolia extract ranging from 500 to 2000 mg/kg. Animals were observed for any symptoms of toxicity continuously for 4 h then after 24 h and finally the number of survivors was recorded after 72 h.
Antitumor activity in EAC-induced ascites tumor in mice
Survival study
The tumor induction and propagation was carried out according to the method described by CitationJagetia and Baliga (2003). The known numbers of viable EAC cells (2.5 × 106 cells/mouse) were injected intraperitoneally into each mouse in an aseptic condition and the day of tumor inoculation was considered as day zero. Twenty-four hours after tumor inoculation the tumor-bearing animals were randomly divided into desired groups of six each and treated with test compound or vehicle.
Group 1 (CMC): Animals were treated with 10 mL/kg CMC (0.5%), i.p.
Group 2 (extract): Animals were treated with 50 mg/kg P. longifolia extract, i.p.
Group 3 (extract): Animals were treated with 100 mg/kg P. longifolia extract, i.p.
Group 4 (Cisplatin): Animals were injected with cisplatin (CP) 3.5 mg/kg, i.p.
The P. longifolia extract was administered for seven days consecutively starting from day 1 of tumor inoculation. Cisplatin, a single dose of 3.5 mg/kg, i.p., was injected on day 1 which served as standard drug. Every third day animals were weighed to assess the tumor growth. The animals were monitored daily for 45 days and mortality was recorded to calculate the MST (mean survival time). The percentage increase in life span (ILS) was calculated by the formula:
Whole blood count
Different sets of animal were used to assess hematological parameters. The experimental design was the same as described in the survival study. Blood was withdrawn on 8th day from retro-orbital plexus of mice. The total white blood cells (WBC), red blood cells (RBC) and haemoglobin content were determined using standard methods (CitationMukherjee, 1990).
Antitumor activity in DLA-induced solid tumor model in mice
Antitumor activity of P. longifolia extract was determined in DLA-induced solid tumor model as per the method of Rajesh Kumar et al., 2002. In brief, DLA cells (1 × 106 cells per mouse) were inoculated subcutaneously into the hind limb of mice. After 24 h mice were randomized and divided into four groups of six each. Group I served as control and received 0.5% CMC, i.p. Group II received 3.5 mg/kg cisplatin, i.p., single dose on the first day. Groups III and IV received P. longifolia extract 50 and 100 mg/kg, i.p., respectively, for seven consecutive days. The diameter of the tumor was measured at five-day intervals for a period of 30 days and tumor volume was calculated using the standard formula
where r1 and r2 represent the radii of the tumor at two different planes.
Effect of P. longifolia extract on in vitro antioxidant activities
DPPH radical scavenging activity
DPPH scavenging activity of P. longifolia extract was determined by incubating equal volumes of different concentrations of P. longifolia extract with 100 µM DPPH in methanol at room temperature. Absorbance was recorded at 517 nm after 20 min using methanol as blank and percentage DPPH radical scavenging was calculated using the formula:
The experiment was carried out in triplicate and ascorbic acid was used as standard (CitationSreejayan & Rao, 1996).
Ferric ion reduction activity
Electron donating capability was evaluated by ferric chloride reduction method. Reaction mixture contained 1 mL of phosphate buffer (pH 7.4), 100 µM Fe3+ and different concentrations of extract in 0.5 mL of PBS. After 3 min incubation, EDTA (100 M) and orthophenanthroline (300 M) were added, reaction was allowed for 10 min at room temperature and absorbance was recorded at 510 nm. Ascorbic acid was used as standard which is equivalent to 100% reduction of ferric ions, comparative reduction of Fe3+ by P. longifolia extract was calculated (CitationKunchandy & Rao, 1987).
Anti-lipid peroxidation
Albino rats (180-200 g) of either sex were used for the study. Animals were anesthetized and perfused transcardially with ice-cold saline after which the brain was collected. The isolated tissue was weighed and 10% homogenate was prepared in 150 mM KCl. Inhibition of lipid peroxidation was determined in rat brain homogenate. The reaction mixture contained 0.1 mL FeCl3 (1 mM), 0.1 mL ascorbic acid (1mM), 0.1mL of KCl (1.5 M), 0.1 mL of various concentrations of P. longifolia extract and 0.3 mL of brain homogenate (10%) in a final volume of 1 mL. After 20 min of incubation at room temperature, the reaction was stopped by addition of 1 mL of 15% TBA, 0.38% TCA and 0.05% BHT solution. Absorbance of supernatant was recorded at 532 nm after heating at 80°C for 15 min and centrifugation at 1000 rpm (CitationRajakumar & Rao, 1994). Anti-lipid peroxidation activity was calculated by the formula
where C is the absorbance of the control and S is the absorbance of the sample. Each experiment was carried out in triplicate and results were expressed as percentage anti-lipid peroxidation activity ± SEM.
Statistical analysis
Data obtained were expressed as mean ± SEM of indicated number of animals. Statistical analysis was carried out using one way ANOVA with Tukey’s post hoc test (GraphPad Prism version 4.03 for Windows, GraphPad Software, California, USA). A value of p < 0.05 was considered to be significant. Graphs were prepared by OriginLab Origin Pro 8.0 (OriginLab Software, Northampton, MA). The daily survival was determined by Kaplan Meir’s equation.
Results
In vitro cytotoxic activity
P. longifolia extract exhibited dose-dependent cell death in both EAC and DLA cells with an IC50 value of 45.77 and 52.52 µg/mL in EAC and DLA, respectively ().
Table 1. In vitro cytotoxic activity of P. longifolia extract on EAC and DLA cells by Trypan blue exclusion assay. EAC/DLA cells (1 × 106) were incubated in 1 mL of PBS containing different concentrations of extract for 3 h at 37°C. After the exposure period, percentage cytotoxicity was calculated using Trypan blue dye. All values are expressed as mean ± SEM. The experiment was performed in triplicate.
Effect on cultured human cancer cells
P. longifolia extract inhibited the proliferation in both the tested human cancer cell lines in a dose-dependent manner. However, the growth inhibition of HeLa cells was greater because of shorter doubling time as compared to MCF-7cells. The IC50 (concentration required to inhibit 50% of cell growth) value of P. longifolia extract in HeLa and MCF-7 cells was observed to be 25.24 and 50.49 µg/mL, respectively (). The percentage of DMSO (0.1%) used in the experiment did not affect the growth of the cells.
Table 2. Effect of P. longifolia extract on proliferation of human cancer cell lines assessed by MTT assay. A fixed number of cells (1 × 104) grown in a 96-well culture plate were exposed to different concentrations of P. longifolia extract for 48 h followed by MTT addition and absorbance was recorded at 540 nm by ELISA reader. The IC50 for standard doxorubicin in HeLa cells was 1.16 µg/mL. All values are expressed as mean ± SEM.
Toxicological study (median lethal dose)
In the toxicity study, no mortality occurred within 72 h under the tested doses. The P. longifolia extract was found safe up to 2000 mg/kg. On the basis of toxicological data, therapeutic doses were selected.
Effect on mean survival time
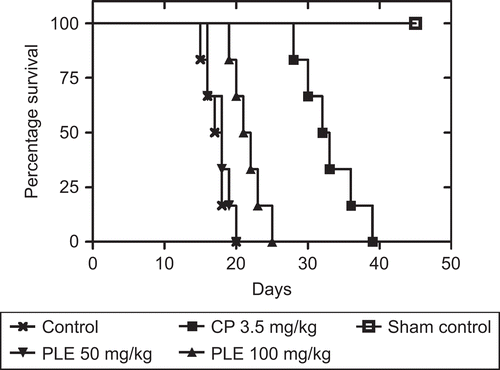
In the EAC vehicle control group (Group 1), the average life span of animals was found to be 17.21 ± 0.93 days. The average life-span of P. longifolia extract treated animals at both the doses (50 and 100 mg/kg) was 18.33 ± 0.65 and 22.28 ± 0.88 days. But only the higher dose was found significant (p < 0.05) when compared with control. The average life span of cisplatin treated mice was found to be 33.02 ± 1.03 days ().
Figure 1. Effect of P. longifolia extract treatment on the Kaplan-Meier estimate of survival of EAC-bearing mice. After 24 h of EAC, inoculation (2.5 × 106 cells/mouse, i.p.), mice were treated with 50 or 100 mg/kg of P. longifolia extract for seven consecutive days or a single dose of cisplatin (3.5 mg/kg) as positive control. Animals were monitored daily for a period of 45 days.
Effect on body weight changes
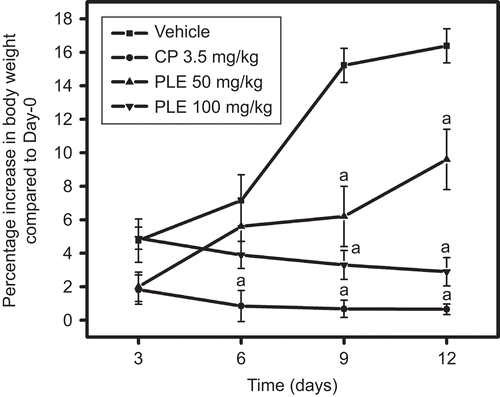
Substantial gain in body weight was observed in vehicle-treated mice with maximum gain of 16.35% on day 12. Cisplatin administration significantly reduced the weight gain as compared to control on all the tested days whereas the reduction in the weight gain was significant on days 9 and 12 as compared to control with P. longifolia extract treatment at both the tested doses ().
Figure 2. Effect of P. longifolia extract on body weight changes in EAC inoculated mice. EAC (2.5 × 106 cells/mouse, i.p.) inoculated mice treated with P. longifolia extract and cisplatin as per the given regimen were weighed on every third day and change in body weight was recorded and percentage increase in body weight was calculated. All values are the mean ± SEM of six mice; ap < 0.05 compared to vehicle treatment.
Effect on hematological parameters
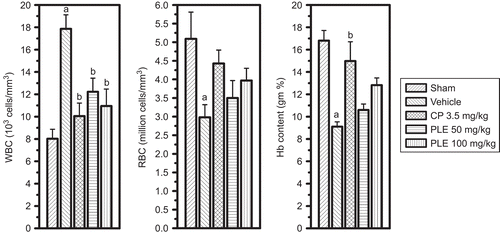
In the EAC vehicle control, the reduction in total RBC, hemoglobin (Hb) content and increase in total WBC count were significant when compared with normal mice. Cisplatin (3.5 mg/kg i.p., single dose) significantly normalized the EAC-induced hematological changes. P. longifolia extract at both the doses (50 and 100 mg/kg) significantly reversed the elevated WBC count. However, the increase in RBC and Hb content was not significant ().
Figure 3. Effect of P. longifolia extract on hematological parameters in EAC challenged mice. The myelotoxicity of EAC-bearing animals (2.5 × 106 cells/mouse, i.p.), receiving P. longifolia extract (50 or 100 mg/kg for 7days), cisplatin (3.5 mg/kg single dose) and vehicle, was assessed by counting the RBC, WBC and hemoglobin content on day 8. All values are the mean ± SEM of six mice; ap <0.05 compared to sham mice; bp <0.05 compared to vehicle treatment.
Effect on DLA-induced solid tumor
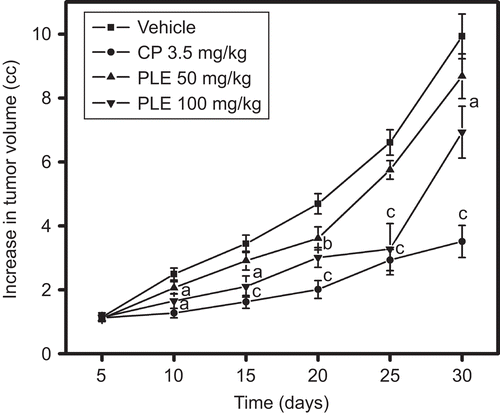
The tumor volume of vehicle-treated animals on day 30 after tumor inoculation was found to be 9.9 cc. The tumor volume was reduced to 8.67 and 6.93 cc by P. longifolia extract administration at the dose of 50 and 100 mg/kg, respectively. Cisplatin significantly reduced the tumor volume to 3.51 cc (). The percentage reduction in the tumor volume of P. longifolia extract-treated animals was found to be 12.68% and 30.21%, whereas cisplatin reduced the tumor volume by 64.55%.
Figure 4. Effect of P. longifolia extract administration on solid tumor growth induced by DLA cells. DLA cells (1 × 106cells) were injected s.c. into a hind limb of each mouse in an aseptic condition. After 24 h of tumor inoculation mice were treated as per the treatment schedule mentioned in the methodology, and tumor volume was measured on every fifth day. On day 30 the tumor volume and weight were recorded. All values are the mean ± SEM of six mice, ap <0.05, bp <0.01 & cp <0.001, compared to vehicle treatment.
Effect on free radical scavenging activity
P. longifolia extract significantly scavenged DPPH radical and inhibited lipid peroxidation in rat brain homogenate with IC50 values of 18.14 and 73.33 µg/mL, respectively. The IC50 values of ascorbic acid and curcumin in DPPH radical scavenging and lipid peroxidation activity were 1.54 and 4.04 µg/mL, respectively. The IC50 values of P. longifolia extract and ascorbic acid in ferric ion reduction were found to be 155.41 and 35.53 µg/mL, respectively ().
Table 3. Free radical scavenging and lipid peroxidation inhibiting activity of P. longifolia extract.
Discussion
Previously, P. longifolia extract has been reported to contain aporphine and azafluorene alkaloids (CitationWu & Duh, 1990), proanthocyanidine trimers, sitosterols, and clerodane diterpenes (CitationPhadnis et al., 1988) which have antitumor activities (CitationZhang et al., 2005; CitationSalatino et al., 2007; CitationShrivastava & Patel, 2007). Present in vivo studies revealed significant reduction in the body weights of EAC-bearing animals at a dose of 100 mg/kg. Another criterion for evaluating antitumor potential is prolongation of life span of tumor-bearing mice (CitationClarkson & Burchenal, 1965) with reversal of the elevated total levels of WBC (CitationOberling & Guerin, 1954).This parameter has also been fulfilled by P. longifolia extract as observed from the results.
To further confirm antitumor activity, the effect of P. longifolia extract on solid tumor was assessed using DLA cells. Significant reduction in tumor volume was observed with P. longifolia extract treatment, which implies inhibition of DLA tumor growth. This indicates that the inhibitory effect of P. longifolia extract is not only due to its local cytotoxic effect but also due to its systemic action.
The short term in vitro cytotoxicity and antiproliferative data also supports the in vivo antitumor activity of P. longifolia extract against EAC and DLA, where P. longifolia extract caused significant cell death and inhibition of cancer cell growth.
Reactive oxygen species have multiple functions (CitationValko et al., 2006) and are implicated in tumor initiation and progression (CitationCzapski & Goldstein, 1990; CitationSagun et al., 2006; CitationNishigori et al., 2004). Depleted endogenous antioxidant enzymes with enhanced free radical generation and MDA are well documented in carcinogenesis (CitationSzatrowski & Nathan, 1991). Many tumor cells have pro-oxidant status and promote oxidative stress. This increases the surviving potential of the cancer cells by inducing mutations, activating redox signaling and stimulating pro-survival factors such as NF κB and AP-1 (CitationSeeram et al., 2005). Antioxidants alter the intracellular redox state, thereby enhancing the effects of cytotoxic therapy. We have observed that P. longifolia extract significantly scavenged DPPH, reduced ferric ion and inhibited lipid peroxidation which proves its antioxidant activity. It was reported that plant-derived extracts containing antioxidant principles showed cytotoxicity toward tumor cells (CitationJiau & Larry, 1977) and antitumor activity in experimental animals (CitationRuby et al., 1995). The cytotoxic and antitumor activity of plant-derived product is either through induction of apoptosis or inhibition of neovascularization (CitationMing et al., 1998). Plants with high phenol content are reported to possess effective antioxidant and antitumor properties (CitationLee et al., 2004) and P. longifolia extract has been found to have high phenol content from the antioxidant studies performed, hence could have antitumor activity.
A phytochemical study on the hexane extract of stem bark of PL has led to the characterization of various clerodane and ent-halimane diterpenes (CitationHara et al., 1995) which are active constituents that are reported to have antitumor activity (CitationSeeram et al., 2005) and the MeOH extract of stem parts of PL have been reported to contain cytotoxic aporphine alkaloid liriodenine (CitationWu & Duh, 1990). The presence of any of these components may be attributed to the antitumor property of the extract. Since the present study focuses on the preliminary antitumor activity of P. longifolia extract, the tumor selective action and characterization of the active component of P. longifolia extract responsible for the activity are yet to be explored.
Acknowledgements
The authors thank Professor Dr. N. Udupa, Principal, Manipal College of Pharmaceutical Sciences for providing all the necessary facilities to carry out this research work.
Declaration of interest
The authors declare no conflict of interest. The authors themselves are responsible for the content and writing of the paper.
References
- Clarkson BD, Burchenal JH (1965): Preliminary screening of anti neoplastic drugs. Prog Clin Cancer 1: 625–629.
- Czapski G, Goldstein S (1990): Superoxide scavengers and SOD or SOD mimics. Adv Exp Med Biol 264: 45–50.
- Hara N, Asaki H, Fujimoto Y, Gupta YK, Singh AK, Sahai M (1995): Clerodane and ent-halimane diterpenes from Polyalthia longifolia. Phytochemistry 38:189–194.
- Jagetia GC, Baliga MS (2003): Modulation of antineoplastic activity of cyclophosphamide by Alstonia scholaris in the Ehrlich ascites carcinoma-bearing mice. J Exp Ther Oncol 3: 272- 282.
- Jiau JL, Larry WO (1977): Overexpression of manganese-containing superoxide dismutase confers resistance to the cytotoxicity of tumor necrosis factor and/or hyperthermia. Cancer Res 57: 1991–1998.
- Kunchandy E, Rao MNA (1987): Effect of curcumin on hydroxyl radical generation through Fenton reaction. Int J Pharm 57: 237–240.
- Lee J, Koo N, Min DB (2004): Reactive oxygen species, aging and antioxidant nutraceuticals. Comp Rev Food Sci Food Safety 3: 21–32.
- Malairajan P, Geetha G, Narasimhan S, Jessi KKV (2006): Analgesic activity of some Indian medicinal plants. J Ethnopharmacol 106: 425–428.
- Ming L, Jill CP, Jingfang JN, Edward C, Brash E (1998): Antioxidant action via p53 mediated apoptosis. Cancer Res 58: 1723–1729.
- Mossman T (1983): Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63.
- Mukherjee KL (1990): Medical Laboratory Technology. New Delhi, Tata McGraw Hill, pp. 224–232.
- Murthy MM, Subramanyam M, Hima BM, Annapurna J (2005): Antimicrobial activity of clerodane diterpenoids from Polyalthia longifolia seeds. Fitoterapia 76: 336–339.
- Newman DJ, Cragg GM, Snader KM (2003): Natural products as a source of new drugs over the period of 1981-2002. J Nat Prod 66: 1022–1037.
- Nishigori C, Hattori Y, Toyokuni S (2004): Role of reactive oxygen species in skin carcinogenesis. Antioxid Redox Signal 6: 561–570.
- Oberling C, Guerin M (1954): The role of viruses in the production of cancer. Adv Cancer Res 2: 353–423.
- OECD (2001): The OECD Guidelines for Testing of Chemicals: 420 Acute Oral Toxicity. Paris, Organization of Economic Co-operation and Development, pp. 1–14
- Phadnis AP, Patwardhan SA, Dhaneshwar NN, Tavale SS, Row TNG (1988): Clerodane diterpenoids from Polyalthia longifolia. Phytochemistry 27: 2899–2901.
- Rajakumar DV, Rao MNA (1994): Antioxidant properties of dehydrozingerone and curcumin in rat brain homogenates. Mol Cell Biochem 140: 73–79.
- Rajeshkumar NV, Joy KL, Kuttan G, Ramsewak RS, Muraleedharan GN, Kuttan R (2002): Antitumour and anticarcinogenic activity of Phyllanthus amarus extract. J Ethnopharmacol 81: 17–22.
- Rastogi RP (1997): Compendium of Indian Medicinal Plants. New Delhi, CSIR.
- Ruby AJ, Kuttan G, Babu KD, Rajashekaran KN, Kuttan R (1995): Antitumor and antioxidant activity of natural curcuminoids. Cancer Lett 94: 79–83.
- Sagun KC, Carcamo JM, Golde DW (2006): Antioxidants prevent oxidative DNA damage and cellular transformation elicited by the over-expression of c-MYC. Mutat Res 593: 64–79.
- Salatino A, Salatino MLF, Negri G (2007): Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J Braz Chem Soc 18: 11–33.
- Seeram NP, Adams LS, Henning SM, Niu Y, Yanjun Zhang Y, Nair MG, Heber D (2005): In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutri Biochem 16: 360–367.
- Sheeja KR, Kuttan G, Kuttan R (1997): Cytotoxic and antitumor activity of berberin. Amala Res Bull 17: 73–76.
- Shrivastava N, Patel T (2007): Clerodendrum and healthcare: An overview. Med Aromatic Plant Sci Biotech 1: 142–150.
- Sreejayan N, Rao MNA (1996): Free radical scavenging activity of curcuminoids. Arzneim-Forsch/Drug Res 46: 169–171.
- Szatrowski T, Nathan C (1991): Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 51: 794–798.
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006): Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biol Interact 160: 1–40.
- Verma M, Singh KS, Shashi Bhushan S, Sharma VK, Dutt P, Kapahi BK, Saxena AK (2008): In vitro cytotoxic potential of Polyalthia longifolia on human cancer cell lines and induction of apoptosis through mitochondrial-dependent pathway in HL-60 cells. Chemico-Biol Interact 171: 45–56.
- Wu YC, Duh CY (1990): Two new natural azafluorene alkaloids and a cytotoxic aporphine alkaloid from Polyalthia longifolia. J Nat Prod 53: 1327–1331.
- Zhang XY, Li WG, Wu YJ, Bai DC, Liu NF (2005): Proanthocyanidin from grape seeds enhances doxorubicin-induced antitumor effect and reverses drug resistance in doxorubicin-resistant K562/DOX cells. Can J Physiol Pharmacol 83: 309–318.