Abstract
Morinda citrifolia Linn (Rubiaceae) is a traditional medicinal herb that has been purported to be beneficial in the treatment of infections due to its immune enhancing properties. However, detailed studies highlighting the effect of different compounds isolated from the plant on the immune system are lacking. In this study, the stimulatory effects of the extracts and fractions of M. citrifolia fruits on important components of the adaptive immune system such as T lymphocytes and B lymphocytes were studied. The effects of the plant extracts on lymphocytes were assessed by in vitro (MTT assay) and in vivo (cell mediated immune response) techniques. Results of the MTT study indicated that the hydroalcoholic (0.5 and 1.0 mg/mL) and aqueous extracts (0.5 and 1.0 mg/mL) significantly (p < 0.05) increased in vitro splenocyte proliferation to the extent of 43.6, 54.5, 32.7, and 36.4%, respectively. Moreover, the hydroalcoholic (200 mg/kg) and the aqueous (200 mg/kg) extracts significantly (p < 0.05) increased the cell-mediated immune response to the extent of 33.52 and 18.56%, respectively. The fractions F I, F II, and F III failed to elicit a significant stimulatory effect on lymphocytes in the in vitro and in vivo studies. The effect of the extractives of M. citrifolia fruits on B-cells was measured by the delayed type hypersensitivity method. The study revealed that the hydroalcoholic extract (200 mg/kg) and fraction F I (40 mg/kg) significantly increased the humoral response to the extent of 33.33 and 35.12%, respectively. The results of this study confirm the cellular and humoral immunostimulant properties of M. citrifolia fruits and justify its usage in traditional medicine.
Introduction
Morinda citrifolia Linn (Rubiaceae), popularly known as “Indian mulberry” or “noni,” is a plant indigenous to India, Burma, China, and the Polynesian islands (CitationKirtikar & Basu, 1933; CitationWang et al., 2002). M. citrifolia fruits have been used traditionally by native Polynesians to treat diabetes, high blood pressure, cancer, injury, arthritis, digestive distress, arteriosclerosis, pain, and senility (CitationKrishnamoorthy & Reddy, 1970; CitationYounos et al., 1990; CitationDerMarderasian, 1999; CitationEiichi, 2003). In addition, M. citrifolia fruits have also been used as a remedy for halitosis, bacterial and helminthic infection, wound healing, menstrual cramps, arthritis, gastric and oral ulcers, toothache, and indigestion. It improves lactation and also acts as a purgative (CitationBhandari, 1985; CitationMcClatchey, 2002; CitationSaludes et al., 2002; CitationNayak et al., 2009).
Several researchers have confirmed that the immunostimulant properties of M. citrifolia significantly contribute to its antitumor potential (CitationHiramatsu et al., 1993; CitationHirazumi et al., 1994, Citation1996; CitationLiu et al., 2001; CitationWang & Su, 2001; CitationJohnson et al., 2002; CitationEiichi, 2003; CitationLin, 2005). Recently, CitationPalu et al. (2008) demonstrated that M. citrifolia juice concentrate (NFJC) stimulated the cannabinoid receptors (CB2). In addition, a decrease in interleukin-4 (IL-4) levels with a concomitant increase in interferon-γ (IFN-γ) levels elicited by NFJC indicated the immunostimulant properties of this plant. However, there are no experimental reports of the effect of this plant on T lymphocytes and B lymphocytes, the major mediators of the adaptive immune response. This prompted us to investigate the effects of the extracts and fractions of M. citrifolia fruits on these important immunological parameters with a view to elucidating the probable mechanisms involved in the immunostimulant effects of this indigenous medicine.
Materials and methods
Dried fruits of M. citrifolia were obtained from M/s Anju Phytochemicals Pvt. Limited (Bangalore, India) and authenticated by Dr. Vinayak Naik, Botanist, at Nicholas Piramal Research Institute, Mumbai, India. An authenticated voucher specimen of the plant (No. 4944) has been deposited. The dried fruits were pulverized in a hammer mill.
Preparation and extraction of the plant material
Aqueous extract
M. citrifolia fruit powder (50 g) was exhaustively extracted with distilled water in a Soxhlet extractor and concentrated in a rotary evaporator (Rotavap; Equitron Roteva, Medica Instrument Mfg. Co.) to obtain 11.85 g (23.7% w/w) of the aqueous extract.
Hydroalcoholic extract
M. citrifolia fruit powder (50 g) was subjected to continuous Soxhlet extraction using 300 mL ethanol–water (1:1) till the powder was completely exhausted. The extract was filtered through Whatman No. 1 filter paper and concentrated under reduced pressure in a rotary evaporator to obtain 12.7 g (25.4% w/w) of the hydroalcoholic extract.
Methods of fractionation to obtain the polysaccharide-rich fraction (fraction I), anthraquinone-rich fraction (fraction II), and alkaloid-rich fraction (fraction III) were employed.
Fraction I
M. citrifolia fruit powder (50 g) was defatted using methanol (300 mL) and extracted with distilled water (300 mL) for 12 h in a Soxhlet extractor. The aqueous extract was concentrated under reduced pressure and poured into 500 mL of acetone. The acetone-insoluble precipitate (1 g) was dissolved in 50 mL of water. Then, 25 mL of 12% w/v aqueous trichloroacetic acid was added and the impurities were filtered off. The filtrate was poured into 500 mL acetone to precipitate the acetone-insoluble polysaccharide-rich component. The precipitate was filtered off under vacuum and air-dried to obtain 3.12 g (6.24% w/w) of fraction I (CitationChintalwar et al., 1999; CitationNair et al., 2004; CitationSchepetkin et al., 2005).
Fraction II
M. citrifolia fruit powder (50 g) was refluxed with 250 mL of a methanol–water (2:3) mixture for 3 h. The resultant extract was acidified by the addition of concentrated hydrochloric acid. Further, 5 mL of a 5% methanol solution of ferric chloride was added and refluxed for 6 h. The mixture was allowed to cool and the anthraquinone-rich component was partitioned into 100 mL of chloroform. The chloroform layer was evaporated to dryness to obtain 0.24 g (0.47% w/w) of fraction II (CitationBrain & Turner, 1975; CitationSu & Ferguson, 2006).
Fraction III
M. citrifolia fruit powder (25 g) was refluxed with 100 mL of ethanol–chloroform (1:3) containing 2% v/v of a strong solution of ammonia for 6 h. The resultant mixture was extracted with three 20 mL portions of 2 N hydrochloric acid. The acid extracts were combined and their pH adjusted to 8.0 by dropwise addition of strong ammonia solution. Chloroform was added and the alkaloid-rich component was extracted into the chloroform layer. The chloroform layer was evaporated to dryness to obtain 0.06 g (0.12% w/w) of fraction III (CitationVishin & Gupta, 1967; CitationDjilani et al., 2006).
Chemicals and reagents
Prednisolone was procured from Wyeth Limited, India. The reagent 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), penicillin, streptomycin, and Rosewell Park Memorial Institute (RPMI)-1640 medium were purchased from S.D. Fine Chemicals Ltd, Mumbai. Fetal calf serum was a gift sample from Glenmark Pharmaceuticals Ltd, Mumbai, India. All other chemicals and reagents were of analytical grade.
Antigen for eliciting a cell mediated immune response
Bacillus Calmette Guerin (BCG) vaccine was selected as the antigen for eliciting a cell mediated immune response, since immunization with BCG is known to induce strong T-cell responses such as localized swelling in the mouse footpad model (CitationLagranderie et al., 1996; CitationChambers et al., 1997). BCG vaccine I.P. manufactured by the Serum Institute of India, Pune, India, was used for antigenically challenging rats. Each vial containing between 10 × 105 and 330 × 105 colony forming units (CFU) was diluted with 4 mL of pyrogen-free sterile saline, and 0.1 mL of this reconstituted solution was immediately used as the antigen for eliciting a cell mediated immune response.
Antigen for eliciting a delayed type hypersensitivity response
Red blood cells (RBCs) can bind to RBC-specific antibodies leading to a visible agglutination reaction (CitationBoyd, 1947). In this context, sheep red blood cells (SRBCs) have been widely employed as antigens for eliciting anti-SRBC antibody titers in rats (CitationSharma et al., 1994; CitationDavis & Kuttan, 2000; CitationTiwari et al., 2004). In the present investigation, we used fresh sheep blood collected in Alsever’s solution from Haffkine Biopharmaceuticals, Mumbai, India as the source of SRBCs for eliciting humoral responses in rats. SRBCs were separated from the blood by centrifugation, and washed three times with pyrogen-free sterile saline and adjusted to a concentration of 5 × 109 cells mL−1 in saline for further use (CitationSharma et al., 1994).
Standard herbal drug
Withania somnifera L. Dunal (Solanaceae) is an indigenous immunostimulant plant that has been reported to enhance the proliferation of lymphocytes and to elicit significant cell mediated and humoral immune responses in rats (CitationDavis & Kuttan, 2002; CitationMalik et al., 2007). Thus, W. somnifera was selected as a standard in our studies to explore the immunostimulant activity of M. citrifolia. A marketed preparation of W. somnifera (Ashvagandha capsules 250 mg) manufactured by Himalaya Drug Co. Private Ltd, India, was used.
Animals
Adult Wistar rats of either sex, weighing 180–200 g, were used in the study. They were kept in standard environmental conditions and fed with a rodent diet and water ad libitum. All the experimental procedures and protocols used in this study were reviewed and approved by the Institutional Animal Ethics Committee (IAEC) of C.U. Shah College of Pharmacy, Mumbai, India, and the pharmacological work was performed as per Committee for the Purpose of Control and Supervision of Experimentation on Animals (CPCSEA) norms.
In vitro study
Isolation of splenocytes
All the procedures were carried out under aseptic conditions. A single cell suspension containing splenocytes was prepared from isolated rat spleen by pressing the spleen between two glass slides. The cell suspension was passed through a 200 mesh stainless steel sieve and then allowed to stand to remove tissue fragments. The cells were washed with RPMI-1640 and centrifuged in a refrigerated centrifuge (Eltek; Lab Enterprises, India) at 5000 rpm for 10 min at 20°C. The red blood cells present in the centrifugate were lysed by the addition of ammonium chloride solution (0.84% w/v). The splenocytes were resuspended in RPMI-1640. The viability of splenocytes was determined by the trypan blue exclusion test (0.2% w/v) and was found to be greater than 95%. The cells were adjusted to a concentration of 1 × 106 cells mL−1 using a hemocytometer (CitationTanaka et al., 1999; CitationGeeta et al, 2002).
Splenocyte proliferation determined by MTT assay
Splenocyte proliferation was determined using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) reagent (CitationCho et al., 2001; CitationOveido-Chavez et al., 2004). Proliferating splenocytes when incubated with MTT reduce yellow-colored MTT to a purple-colored insoluble formazan (CitationMosmann, 1983), which can be determined colorimetrically. The isolated splenocytes (1 × 106 cells/mL, 100 μL) were incubated in a 96-well culture plate with 100 μL of RPMI-1640 supplemented with streptomycin (100 μg mL−1), penicillin (100 IU mL−1), and 40 μL of fetal calf serum. Different concentrations of the extracts (0.25–1.0 mg mL−1) and fractions (F I: 0.05–0.2 mg mL−1, F II: 0.005–0.02 mg mL−1, F III: 0.001–0.004 mg mL−1) were added to each well and the culture plates were incubated at 37°C for 48 h under CO2. At the end of the incubation period, 20 μL of MTT solution (10 mg mL−1) was added to all the wells and incubation was continued for 4 h. The formazan produced was dissolved by adding 10 μL of the lysing agent (sodium dodecyl sulfate 20% w/v and dimethyl formamide 50% w/v in distilled water) to each well. After 30 min incubation, the absorbance was read at 570 nm using an enzyme-linked immunosorbent assay (ELISA) microplate reader (Lisa-5; Transasia, India).
In vivo study
Treatment
The rats were divided into 18 groups, each group containing six rats. Two control groups were employed in the study. Rats of control group I were administered only the vehicle (0.1% sodium carboxy methyl cellulose solution), and their responses reflected the normal immune responses of untreated rats. Rats of vehicle control group II were co-administered prednisolone (5 mg/kg), leading to the suppression of their immune responses.
Selection of dose range for the study
Antitumor activity of Morinda citrifolia fruit juice and the polysaccharide-rich fraction has been reported in the dose ranges of 3–20 mg/mouse and 0.8–1.6 mg/mouse, respectively (CitationHirazumi & Furusawa, 1999). Since immunostimulation was reported to be the underlying mechanism of the antitumor activity, the dose range for the in vivo evaluation of the extracts and fractions in the current investigation was decided after taking into consideration the above mentioned reported doses. Further, the aqueous extract of Morinda citrifolia fruits has also been reported to possess a significant anti-inflammatory effect at the intraperitoneal dose of 200 mg kg−1 (CitationMcKoy et al., 2002). This prompted us to evaluate both the aqueous and hydroalcoholic extracts orally at doses of 50, 100, and 200 mg kg−1 in rats. The doses of the three fractions tested were calculated based on their yields. Fraction F I was administered orally at doses 10, 20, and 40 mg kg−1. Investigation of the activity of fractions F II and F III was carried out in the dose ranges 0.25–1.0 mg kg−1 and 0.05–0.2 mg kg−1, respectively. Weighed quantities of the extracts and fractions were suspended in vehicle (0.1% sodium carboxy methyl cellulose solution) and administered orally for 5 days. Immunosuppression was induced by co-administering prednisolone (5 mg kg−1) orally to all the treatment groups for 5 days (CitationZiauddin et al., 1996; CitationSharma & Ray, 1997).
The standard group was orally administered W. somnifera extract (100 mg kg−1) and prednisolone (5 mg kg−1) for 5 days (CitationZiauddin et al., 1996).
Cell mediated immune response
The cellular immune response was assayed by the footpad reaction method in rats (CitationSharma et al., 1994; CitationTiwari et al., 2004). The rats were dosed orally as per the protocol for 5 days. On the third day of the study, all the rats were primed by injecting 0.1 mL of the antigen BCG in the subplantar region of the right hind footpad. The animals were challenged on the 10th day of the study by injecting the same amount of BCG into the subplantar region of the left hind footpad. The difference in the left footpad volume before and 24 h after administering the antigen was measured plethysmometrically (Digital Plethysmometer; Panlab, Spain) and expressed as mean percent increase in paw volume.
Humoral immune response
The rats were dosed orally as per the protocol for 5 days. The rats were primed on the third day by an intraperitoneal injection of 100 μL of sheep SRBC (5 × 109 mL−1). Blood samples were collected from the anesthetized rats on the 10th day by retro-orbital puncture. The anti-sheep anti-SRBC titer of rat serum was estimated using the hemagglutination technique (CitationBoyd, 1947). Serial two-fold dilutions of serum samples were made in 100 μL of normal saline containing 0.1% w/v bovine serum albumin (BSA) in microtiter wells. Next, 100 μL of 0.1% SRBC suspension in phosphate buffered saline (count adjusted to 5 × 109 mL−1) was added to each well. The microtiter plates were incubated for 4 h at 37°C and observed for hemagglutination at the end of the incubation period (CitationSharma et al., 1994; CitationDikshit et al., 2000; CitationJoharapurkar et al., 2003). Minimum serum dilution (1:2) was ranked as 1 and subsequent dilutions were expressed in a graded manner (1–15). The value of the highest serum dilution showing hemagglutination was taken as the antibody titer of that sample. The mean ranks of the different treatment groups were statistically compared with the mean ranks of the vehicle control groups I and II.
Statistical analysis
Results are expressed as mean ± SEM. The data was analyzed for statistical significance by one-way analysis of variance (ANOVA) followed by Dunnett’s t-test for comparison with the control group. The difference was considered to be significant at the 5% level (p < 0.05).
Results
In vitro
Splenocyte proliferation determined by MTT assay
The effect of the extracts and fractions of M. citrifolia fruits on the proliferation of splenocytes was studied in vitro and the data is presented in .
Figure 1. Effect of extracts and fractions of Morinda citrifolia fruits on splenocyte proliferation. Numbers written above bar graphs indicate concentrations in mg mL−1 of M. citrifolia fruit extractives, namely aqueous and hydroalcoholic extracts, fractions F I, F II, and F III. *, A slight and insignificant decrease in proliferation of isolated splenocytes; **, a significant increase in proliferation of isolated splenocytes.
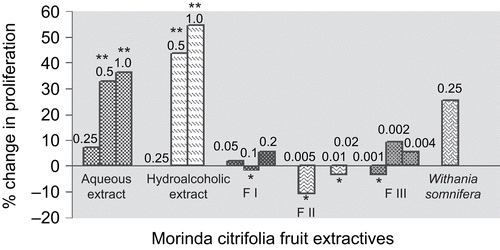
The aqueous extract revealed a slight but insignificant proliferative effect to the extent of 7.5% on isolated T-lymphocytes when tested at the concentration 0.25 mg mL−1. Increasing the concentration of the aqueous extract of Morinda citrifolia fruits to 0.5 and 1.0 mg mL−1 significantly (p < 0.05) increased the in vitro proliferation of splenocytes by 32.7 and 36.4%, respectively. The hydroalcoholic extract failed to stimulate splenocyte proliferation at the concentration of 0.25 mg mL−1. However, it significantly (p < 0.05) increased splenocyte proliferation to the extent of 43.6 and 54.5% when tested at the concentrations 0.5 and 1.0 mg mL−1, respectively. The fractions F I, F II, and F III failed to significantly increase in vitro splenocyte proliferation at all the concentrations tested. Although the standard herbal drug W. somnifera induced the proliferation of T lymphocytes to the extent of 25.5%, this proliferation was not statistically significant.
In vivo
Cell mediated immune response
The extracts and bioactive fractions were evaluated for their effect on the cell mediated immune (CMI) response in prednisolone-induced immunocompromised rats (). Prednisolone (5 mg kg−1 per day, p.o.) significantly (p < 0.05) suppressed immunity in rats as evidenced by the increase in paw volume to the extent of only 2.39% in immunocompromised rats (vehicle control II). As against this, rats that were not administered prednisolone (vehicle control I) demonstrated a 10.01% increase in paw volume.
Table 1. Effect of administration of extracts and fractions of fruits of Morinda citrifolia on cell mediated immune response in rats.
The hydroalcoholic extract at the dose of 50, 100, and 200 mg kg−1 demonstrated a 9.54, 16.73, and 33.52% increase in paw edema, while the aqueous extract elicited an increase in paw edema to the extent of 3.75, 6.68, and 18.56%, respectively. Statistical evaluation of the data revealed that pretreatment of the rats with the hydroalcoholic and aqueous extracts at the dose 200 mg kg−1 significantly (p < 0.05) reversed prednisolone-induced immunosuppression as compared to vehicle control I rats. In the same study, the fractions F I (40 mg kg−1), F II (1.0 mg kg−1), and F III (0.2 mg kg−1) of M. citrifolia fruits demonstrated an increase in paw edema to the extent of 13.89, 11.6, and 15.43%, respectively. However, this increase was not significant as compared to the response elicited by vehicle control I rats. Administration of the standard herbal drug W. somnifera resulted in a significant increase (p < 0.05) in paw volume to the extent of 21.82%.
Humoral immune response
The effect of the extracts and bioactive fractions of M. citrifolia on the humoral immune response of prednisolone-induced immunocompromised rats is presented in . The ranks of the highest serial dilution of sera showing hemagglutination were recorded and ranged from 1 to 15. Similar to the effect on CMI response, prednisolone significantly reduced the anti-SRBC antibody titer of immunocompromised rats (vehicle control II) to 5.0 as against the titer of 8.0 in normal rats (vehicle control I).
Table 2. Effect of administration of extracts and fractions of fruits of Morinda citrifolia on anti-sheep red-blood cell (SRBC) antibody titer in rats.
The anti-SRBC antibody titer elicited by 200 mg kg−1 of the hydroalcoholic extract (12.0) and 40 mg kg−1 of the fraction F I (12.33) was significantly (p < 0.05) greater than that of vehicle control I rats (8.0). However, the hydroalcoholic extract and fraction F I failed to elicit a significant increase in the anti-SRBC antibody titers at lower doses. The standard herbal drug W. somnifera also significantly elevated (p < 0.05) the anti-SRBC titer to 12.33. At all doses tested, the aqueous extract and the fractions F II and F III of M. citrifolia fruits failed to elicit an increase in the anti-SRBC antibody titers of rats.
Discussion
Several scientific studies have indicated that M. citrifolia fruits show immunostimulant potential (CitationHiramatsu et al., 1993; CitationHirazumi et al., 1994, Citation1996; CitationLiu et al., 2001; CitationWang & Su, 2001; CitationJohnson et al., 2002; CitationEiichi, 2003; CitationLin, 2005; CitationPalu et al., 2008). However, there is a lacuna with respect to the investigation of this plant on the responses of T-cells and B-cells, which are important mediators of immune function. Hence, the present study was directed toward exploring the effect of M. citrifolia fruit extracts and fractions on T-cell and B-cell responses using in vitro and in vivo methodologies. Studies conducted by CitationHirazumi and Furusawa (1999) on the polysaccharide-rich fraction of M. citrifolia fruits for its antitumor effects have confirmed the immunostimulant properties of this fraction. Anthraquinones of M. citrifolia fruits have also been investigated for their antitumor potential. However, their mechanism of action has not been elucidated (CitationHiramatsu et al., 1993). Further, alkaloids of Morinda citrifolia are an important class of phytoconstituents that have been reported to possess immunostimulant potential (CitationChan-Blanco et al., 2006; CitationTakashima et al., 2007). Hence, in addition to the aqueous and hydroalcoholic extracts, the polysaccharide, anthraquinone, and alkaloid fractions were also considered as candidates for the evaluation of immunostimulant activity.
The extracts and fractions of M. citrifolia fruits were evaluated for their role in enhancing the proliferation of isolated splenocytes, which are a rich source of T-cells and B-cells (CitationKralovec et al., 2007). A significant splenocyte proliferative effect was elicited by the aqueous and hydroalcoholic extracts at 0.5 and 1.0 mg mL−1, indicating that the extracts had a mitogenic effect on the lymphocytes. However, the fractions obtained from M. citrifolia fruits were ineffective in stimulating the proliferation of splenocytes. This study provides a rationale for its traditional use as an immunostimulant and paves the way for further evaluation of the plant extractives using in vivo methodologies.
The literature survey indicated that studies pertaining to the immunostimulant potential of M. citrifolia have focused largely on its cytotoxic effects on tumor cell lines and cytokine modulation (CitationPotterat & Hamburger, 2007). Studies conducted by CitationPalu et al. (2008) on isolated splenocytes incubated with M. citrifolia indicated that IL-4 production was suppressed while IFN-γ production was elevated. This probably accounts for its modulatory action on the immune system. However, its role in influencing critical aspects of immunity such as cell-mediated and humoral responses has not been investigated. In light of this, our studies were directed toward exploration of the effects of the extractives of M. citrifolia fruits on cell-mediated and humoral immune responses in immunocompromised rats. Prednisolone was selected for inducing immunosuppression, since it had been used previously for this purpose in studies pertaining to immunomodulatory plants (CitationZiauddin et al., 1996; CitationSharma & Ray, 1997).
An immune response is initiated upon exposure of the animal to a suitable antigen. Intraperitoneal injection of Bacillus Calmette Guerin (BCG), a reported T-cell stimulant, led to the recruitment of T lymphocytes and macrophages at the site of injection, leading to an increase in rat paw volume, and was used as a parameter for evaluating the cell mediated immune (CMI) response in vivo (CitationLagranderie et al., 1996; CitationChambers et al., 1997; CitationFulzele et al., 2002; CitationMediratta et al., 2002; CitationVan Crevel et al., 2002). In our studies carried out to evaluate the CMI response, the hydroalcoholic and the aqueous extracts reversed prednisolone-induced immunosuppression and induced a significant (p < 0.05) increase in the rat paw volume as compared to vehicle control group I. However, the fractions were unable to overcome the immunosuppressive effects of prednisolone at all the doses tested.
The effect of the extractives of M. citrifolia fruits on B-cells was evaluated by the hemagglutination technique (CitationDavis & Kuttan, 2000; CitationJoharapurkar et al., 2003; CitationHonda et al., 2005; CitationPrasad et al., 2006). The results of our study indicated that the hydroalcoholic extract (200 mg kg−1) and the fraction F I (40 mg kg−1) significantly (p < 0.05) enhanced the antibody titer in immunocompromised animals to the extent of 33.33 and 35.12%, respectively. The aqueous extract as well as the fractions F II and F III failed to elevate the antibody titer in immunocompromised animals.
The immunostimulant activity of polysaccharides obtained from plants has been well documented (CitationOoi & Liu, 2000; CitationTziabanos, 2000). This accounts for the immunostimulant activity of fraction F I. However, the studies failed to reveal the biological efficacy of fractions F II and F III. The plausible explanation could be that the multitude of compounds found in the aqueous and hydroalcoholic extracts (including components of fractions F I, F II, and F III) may exert a synergistic effect resulting in greater immune stimulatory potential of the fruits (CitationBurger et al., 1997).
Thus, the results of the present study indicate that M. citrifolia fruits stimulate major components of the adaptive immune system, namely, the T-cells and B-cells. This probably accounts for the growing popularity of the plant as an immunostimulant. At the same time, clinical investigations of its role in modulating diseases with an immune component need to be conducted in order to justify the widespread usage of the plant M. citrifolia in alternative systems of medicine.
Acknowledgement
We also extend our sincere thanks to Dr. Arvind Jha, GM, Medical & Clinical Services, Shreyas Life Sciences Pvt. Ltd., for his technical support and guidance.
Declaration of interest
The authors thank the All India Institute of Technical Education (AICTE), India, for funding this project.
References
- Bhandari C (1985): Vanaushadhi Chandroday, An Encyclopedia of Indian Botanies and Herbs (Part I). Varanasi, Chaukhambha Sanskrit Samsthan, pp. 123.
- Boyd W (1947): Fundamentals of Immunology. New York, Interscience Publishers, pp. 227.
- Brain K, Turner T (1975): The Practical Evaluation of Phytopharmaceuticals. Bristol, Wright Scientechnica Publication, pp. 106.
- Burger R, Torres A, Warren R, Caldwell V, Hughes B (1997): Echinacea-induced cytokine production by human macrophages. Int J Immunopharmacol 19: 371–379.
- Chambers M, Marshall G, Wangoo A, Bune A, Cook H, Shaw R, Young D (1997): Differential responses to challenge with live and dead Mycobacterium bovis Bacillus Calmette-Guerin. J Immunol 158: 1742–1748.
- Chan-Blanco Y, Vaillant F, Perez A, Reynes M, Brillouet J, Brat P (2006): The noni fruit (Morinda citrifolia L.): a review of agricultural research, nutritional and therapeutic properties. J Food Comp Anal 19: 645–654.
- Chintalwar G, Jain A, Sipahimalani A, Banerji A, Sumariwala P, Ramakrishnan R, Sainis K (1999): An immunologically active arabinogalactan from Tinospora cordifolia. Phytochemistry 52: 1089–1093.
- Cho J, Nam K, Kim A, Park J, Yoo E, Baik, K, Yu Y, Park M (2001): In vitro and in vivo immunomodulatory effects of syringin. J Pharm Pharmacol 53: 1287–1294.
- Davis L, Kuttan G (2000): Immunomodulatory activity of Withania somnifera. J Ethnopharmacol 71: 193–200.
- Davis L, Kuttan G (2002): Effect of Withania somnifera on cell mediated immune responses in mice. J Exp Clin Cancer Res 21: 585–590.
- DerMarderasian A (1999): Morinda. In: Guide to Popular Natural Products. St. Louis, MO, Facts and Comparisons Publishing Group, pp. 160.
- Dikshit V, Damre A, Kulkarni K, Gokhale A, Saraf M (2000): Preliminary screening of immunocin for immunomodulatory activity. Indian J Pharm Sci 62: 257–260.
- Djilani A, Legseir B, Soulmani R, Dicko A, Younos C (2006): New extraction technique for alkaloids. J Braz Chem Soc 17: 518–520.
- Eiichi F (2003): Anti-cancer activity of noni fruit juice against tumors in mice. In: Proceedings of the 2002 Hawaii Noni Conference, College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa, pp. 23–24.
- Fulzele S, Bhurchandi P, Kanoje V, Joshi S, Dorle A (2002): Immunostimulant activity of Ashtamangal ghrita in rats. Indian J Pharmacol 34: 194–197.
- Geeta S, Ram M, Singh V, Ilavazhagan G, Sawhney R (2002): Anti-oxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides) - an in vitro study. J Ethnopharmacol 79: 373–378.
- Hiramatsu T, Imoto M, Koyano K, Umezawa K (1993): Induction of normal phenotypes in RAS-transformed cells by damnacanthal from Morinda citrifolia. Cancer Lett 73: 161–166.
- Hirazumi A, Furusawa E, Chou S, Hokama Y (1994): Anticancer activity of Morinda citrifolia on intraperitoneally implanted Lewis lung carcinoma in syngenic mice. Proc West Pharmacol Soc 37: 145–146.
- Hirazumi A, Furusawa E, Chou S, Hokama Y (1996): Immunomodulation contributes to the anticancer activity of Morinda citrifolia (noni) fruit juice. Proc West Pharmacol Soc 39: 7–9.
- Hirazumi A, Furusawa E (1999): An immunomodulatory polysaccharide-rich substance from the fruit juice of Morinda citrifolia (noni) with antitumor activity. Phytother Res 13: 380–387.
- Honda S, Miyamoto A, Shibuya A (2005). Fcα/µR, a novel Fc receptor for innate antibodies IgM and IgA. Int Cong Series 1285: 78–83.
- Joharapurkar A, Zambad S, Wanjari M, Umathe S (2003): In-vivo evaluation of antioxidant activity of alcoholic extract of Rubia cordifolia Linn. and its influence on ethanol-induced immunosuppression. Indian J Pharmacol 35: 232–236.
- Johnson A, Hemscheidt S, Csiszar W (2002): Cytotoxicity of water and ethanol extracts of Morinda citrifolia (L.) against normal epithelial and breast cancer cell lines. In: Proceedings of the 2002 Hawaii Noni Conference, College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa, p. 22.
- Kirtikar K, Basu B (1933): Indian Medicinal Plants, Vol. II. Delhi, Periodical Expert Book Agency, pp. 1295.
- Kralovec J, Metera K, Kumar J, Watson L, Girouard G, Guan Y, Carr R, Barrow C, Ewart H (2007): Immunostimulatory principles from Chlorella pyrenoidosa - Part 1: Isolation and biological assessment in vitro. Phytomedicine 14: 57–64.
- Krishnamoorthy N, Reddy S (1970): Preliminary phytochemical and pharmacological study of Morinda citrifolia Linn. Antiseptic 67: 167–171.
- Lagranderie M, Balazuc A, Deriaud E, Leclerc C, Gheorghiu M (1996): Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect Immun 64: 1–10.
- Lin K (2005): Ethnobotanical study of medicinal plants used by the Jah hut people in Malaysia. Indian J Med Sci 59: 156–161.
- Liu G, Bode A, Ma W, Sang S, Ho C, Dong Z (2001): Two novel glycosides from the fruits of Morinda citrifolia (noni) inhibit AP-1 transactivation and cell transformation in the mouse epidermal JB6 cell line. Cancer Res 61: 5749–5756.
- Malik F, Singh J, Khajuria A, Suri A, Satti N, Singh S, Kaul K, Kumar A, Bhatia A, Qazi G (2007): A standardized root extract of Withania somnifera and its major constituent withanolide-A elicit humoral and cell-mediated immune responses by up regulation of Th1-dominant polarization in BALB/c mice. Life Sci 80: 1525–1538.
- McClatchey W (2002): From Polynesian healers to health food stores: changing perspectives of Morinda citrifolia (Rubiaceae). Integr Cancer Ther 1: 110–120.
- McKoy M, Thomas E, Simon O (2002): Preliminary investigation of the anti-inflammatory properties of an aqueous extract from Morinda citrifolia (noni). Proc West Pharmacol Soc 45: 76–78.
- Mediratta P, Sharma R, Singh S (2002): Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. J Ethnopharmacol 80: 15–20.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63.
- Nair R, Rodriguez S, Ramachandran R, Alamo A, Melnick S, Escalon E (2004): Immune stimulating properties of a novel polysaccharide from the medicinal plant Tinospora cordifolia. Int Immunopharmacol 4: 1645–1659.
- Nayak S, Sandiford S, Maxwell A (2009): Evaluation of the wound healing activity of ethanol extract of Morinda citrifolia L. leaf. Evid Based Complement Alternat Med 6: 351–356.
- Ooi V, Liu F. (2000): Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr Med Chem 7: 715–729.
- Oveido-Chavez I, Ramirez-Apan T, Soto-Hernandez M, Martinez-Vazquea M (2004): Principles of the bark of Amphipterygium adstringens (Julianaceae) with anti-inflammatory activity. Phytomedicine 11: 436–445.
- Palu A, Hirazumi A, West B, Deng S, Jensen J, White L (2008): The effects of Morinda citrifolia L. (noni) on the immune system: Its molecular mechanisms of action. J Ethnopharmacol 115: 502–506.
- Potterat O, Hamburger M (2007): Morinda citrifolia (noni) fruit – phytochemistry, pharmacology, safety. Planta Med 73: 191–199.
- Prasad V, Jain V, Dorle A (2006): Evaluation of Momordica charantia ghrita for immunomodulatory activity. J Plant Sci 1: 80–85.
- Saludes J, Garson M, Franzblau S, Aguinaldo A (2002): Antitubercular constituents from the hexane fraction of Morinda citrifolia Linn. (Rubiaceae). Phytother Res 16: 683–685.
- Schepetkin I, Faulkner C, Nelson-Overton L, Wiley J, Quinn M (2005): Macrophage modulatory activity of polysaccharides isolated from Juniperus scopolorum. Int Immunopharmacol 5: 1783–1799.
- Sharma M, Rao C, Duda P (1994): Immunostimulatory activity of Picrorhiza kurroa leaf extract. J Ethnopharmacol 41: 185–192.
- Sharma S, Ray S (1997): Effect of herbal preparation on immune response of immunosuppressed mice. Indian J Physiol Pharmacol 41: 293–296.
- Su S, Ferguson N (2006): Extraction and separation of anthraquinone glycosides. J Pharm Sci 62: 899–901.
- Tanaka T, Suguira H, Inaba R, Nishikawa A, Murukami A, Koshimizu K, Ohigashi H (1999): Immunomodulatory action of citrus auraptene on macrophage functions and cytokine production of lymphocytes in female BaLB/c mice. Carcinogenesis 20: 1471–1476.
- Takashima J, Ikeda Y, Komiyama K, Hayashi M, Kishida A, Ohsaki A (2007): New constituents from the leaves of Morinda citrifolia. Chem Pharm Bull 55: 343–345.
- Tiwari U, Rastogi B, Thakur S, Jain S, Jain N, Saraf D (2004): Studies on the immunomodulatory effects of Cleome viscosa. Indian J Pharm Sci 66: 171–176.
- Tziabanos A (2000): Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin Microbiol Rev 13: 523–533.
- Van Crevel R, Ottenhoff T, Van der Meer J (2002): Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev 15: 294–309.
- Vishin ML, Gupta D (1967): Estimation of alkaloids of Kurchi by non-aqueous titration. Indian J Pharm Sci 29: 3.
- Wang M, Su C (2001): Cancer preventive effect of Morinda citrifolia (noni). Ann NY Acad Sci 952: 161–168.
- Wang M, West B, Jensen C, Nowicki D, Su C, Palu A, Anderson G (2002): Morinda citrifolia (noni): A literature review and recent advances in noni research. Acta Pharmacol Sin 23: 1127–1141.
- Younos C, Rolland J, Fluerentin M, Lanhers R, Misslin R, Mortier F (1990): Analgesic and behavioural effects of Morinda citrifolia. Planta Med 56: 430–434.
- Ziauddin M, Phansalkar N, Patki P, Diwanay S, Patwardhan B (1996): Studies on the immunomodulatory effects of Ashwagandha. J Ethnopharmacol 50: 69–76.