Abstract
This study demonstrated that the aqueous extracts of plants employed in Mexican traditional medicine for the treatment of cardiovascular diseases are able to modify the tone of arterial smooth muscle. Agastache mexicana (Kunth) Lint & Epling (Labiatae), Chenopodium murale L. (Chenopodiaceae), Chirantodendron pentadactylon Larreat (Sterculiaceae), Dracocephalum moldavica L. (Labiatae), Psittacanthus calyculatus G. Don (Loranthaceae), Prunus serotina ssp. capuli (Cav. ex Spreng) McVaugh (Rosaceae), and Sechium edule Sw. (Cucurbitaceae) contain secondary metabolites that promote vascular relaxation and display antioxidant activities. As expected, their antioxidant effects showed a significant correlation with the polyphenolics content. However, a lower correlation was found between the antioxidant activity and the maximum vasodilatory effect, suggesting that the vasodilatation elicited by the plant extracts could be only partly attributed to their antioxidant properties. The extract of P. calyculatus, which displayed a maximum vasorelaxant effect that was higher than that of acetylcholine, induced endothelium-dependent vasodilatation. Futhermore, the vasorelaxant response to the P. calyculatus extract was reduced after adding an inhibitor of soluble guanylate cyclase activity, providing evidence that the NO/cGMP pathway is involved. On the other hand, the extracts of Bocconia frutescens L. (Papaveraceae), Magnolia grandiflora L. (Magnoliaceae), and Solanum rostratum Dunal (Solanaceae) induced concentration-dependent contraction of rat aortic rings, suggesting that these plants have potential health benefits for the treatment of ailments such as venous insufficiency. The pharmacological activities of the extracts studied provide scientific support for their ethnomedical use.
Introduction
Epidemiological studies performed during the past decade have shown that cardiovascular diseases are the main cause of death and disability in Mexico (CitationVelázquez et al., 2007). In fact, atherosclerotic ischemic heart disease is the second leading cause of general mortality in our country due to the growing prevalence of atherosclerotic risk factors (CitationMeaney et al., 2007). Vascular endothelium synthesizes and releases a broad spectrum of vasoactive substances, and it plays a fundamental role in the regulation and maintenance of overall cardiovascular homeostasis. Increasing evidence suggests that alterations in endothelial function contribute to the pathogenesis and clinical expression of cardiovascular disease, and one of the major causes of endothelial dysfunction is oxidative stress (CitationVita & Keaney, 2002). Therefore, novel therapeutic strategies that combine vascular smooth muscle relaxation and antioxidative action hold great potential for the prevention and treatment of atherosclerotic vascular disease.
Medicinal plants are an important element of traditional medicine in Mexico, and many are regarded as part of the cultural heritage from pre-Hispanic and colonial times (CitationDe la Cruz, 1991; CitationHernández, 1959; CitationSahagún, 1979). A recent documental and ethnomedical survey of medicinal plants grown in Mexico and widely used by the Mexican population revealed that the following species are highly valued for treating cardiovascular ailments, including atherosclerotic ischemic heart disease, coronary artery disease, and hypertension, among others: Agastache mexicana (Kunth) Lint & Epling (Labiatae), Bocconia frutescens L. (Papaveraceae), Chenopodium murale L. (Chenopodiaceae), Chirantodendron pentadactylon Larreat (Sterculiaceae), Dracocephalum moldavica L. (Labiatae), Magnolia grandiflora L. (Magnoliaceae), Psittacanthus calyculatus G. Don (Loranthaceae), Prunus serotina ssp. capuli (Cav. ex Spreng) McVaugh (Rosaceae), Sechium edule Sw. (Cucurbitaceae), and Solanum rostratum Dunal (Solanaceae). includes local names, ethnomedical uses, part of plant employed, mode of preparation, and references of ethnobotanical information on these plants. It is worth mentioning that the fruits of P. serotina and S. edule are also part of the Mexican diet. A literature search of these 10 plants showed that, even for those most widely used in Mexican traditional medicine, phytochemical and pharmacological data are insufficient for understanding their therapeutic profile. Thus, the purpose of this study was to screen the vasoactive and antioxidant activities of aqueous extracts prepared from these plants. In addition, considering that many plants contain a variety of polyphenols, and current evidence strongly suggests that these compounds contribute significantly to the treatment and prevention of cardiovascular diseases due to their antioxidant and vasorelaxing properties, in the present work the total phenolic and flavonoid contents of the extracts were quantified. Additionally, the presence of some common flavonoids was determined.
Table 1. Local names, ethnomedical uses, part of plant employed, mode of preparation, and references of ethnobotanical information on the plant species.
Materials and methods
Chemicals
Phenolic acids, flavonoids, and all reagents employed in the rat aorta and antioxidant assays were obtained from Sigma (St. Louis, MO, USA). All salts, other reagents, and solvents were obtained from J.T. Baker (Phillipsburg, NJ, USA) or Sigma.
Plant material
All plant samples were obtained in August 2004. Specimens of A. mexicana (101.6 g), D. moldavica (114.4 g), M. grandiflora (130.1 g), and C. pentadactylon (100.2 g) were purchased in Mercado de Sonora in Mexico City, an important public market famous for selling plants with medicinal uses. A. mexicana, D. moldavica, and M. grandiflora specimens were whole plants that included flowers, leaves, and stems, which allowed taxonomic identification. C. pentadactylon specimens were flowers. It is worth mentioning that this species constitutes a monotypic genus whose flowers possess a very characteristic morphology. Aerial parts of C. murale (156.7 g), P. serotina (13.2 g), S. edule (20.0 g), and S. rostratum (164.3 g) were collected in different localities of the State of Querétaro (Mexico). Stems and leaves of B. frutescens (66.8 g) were collected in the State of Michoacán (Mexico), and the stems, leaves, and flowers of P. calyculatus (49.0 g) were obtained from the branch of one of its host plants, Prosopsis laevigata (mesquite) grown in Querétaro (Mexico). Voucher specimens (A. mexicana voucher no. 6627; B. frutescens voucher no. 6535; C. pentadactylon voucher no. 6630; C. murale voucher no. 543; D. moldavica voucher no. 6626; M. grandiflora voucher no. 6628; P. serotina voucher no. 825; P. calyculatus voucher no. 6375a; S. edule voucher no. 836b; and S. rostratum voucher no. 558), identified by Dr. M. Martínez and Dr. L. Hernández-Sandoval, have been deposited in the Ethnobotanical Collection of the Herbarium of Querétaro “Dr. Jerzy Rzedowski” QMEX, located at the Faculty of Natural Sciences, University of Querétaro, Mexico.
Extraction
The collected plant material (50 g) was dried in an oven (45°C), ground into powder (2 mm mesh size), and extracted by digestion with water at 60°C for 24 h. The aqueous extracts were lyophilized and stored at 4°C for later use. The extract yield percentages (w/w) in terms of dry starting material were: A. mexicana (25.2%), B. frutescens (21.4%), C. murale (16.1%), C. pentadactylon (11.7%), D. moldavica (22.6%), M. grandiflora (20.4%), P. calyculatus (22.6%), P. serotina (19.1%), S. edule (12.5%), and S. rostratum (9.8%).
Isolated rat aortic rings assay
All experiments were performed in accordance with the Mexican Official Standard NOM-062-ZOO-1999 for the production, care, and use of laboratory animals (CitationNorma Oficial Mexicana, 2001). Male Wistar rats (275–325 g) were anesthetized with chloroform and sacrificed by decapitation. The descending thoracic aorta was removed and placed in ice-cold oxygenated Krebs–Henseleit solution of the following composition: 126.8 mM NaCl, 5.9 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 30 mM NaHCO3, and 5 mM d-glucose (pH 7.4). Then, the aorta was immediately flushed with Krebs–Henseleit solution to prevent intravascular clot formation. The aorta was dissected free of adipose and connective tissue and cut at 4- to 5-mm intervals into rings. The aortic rings were mounted between stainless steel hooks and suspended in water-jacketed, 7 mL organ baths containing oxygenated (95% O2 and 5% CO2) Krebs–Henseleit solution at 37°C. The tissues were allowed to equilibrate for 60 min under a resting tension of 1.5 g. During this period, the bathing medium was exchanged every 15 min. After final adjustment of the passive resting tension to 1.5 g, aortic segments were contracted with 100 mM KCl. Once a stable contractile tone was reached, the bathing medium was exchanged to restore a resting tension of 1.5 g. After that, the tissues were contracted with 1 μM l-phenylephrine; the developed force of contraction was recorded, and this contraction was defined as 100%. Then acetylcholine (ACh) and the extracts dissolved in deionized water were added to the organ bath at final concentrations of 0.2 ng/mL–2 mg/mL and 1 μg/mL–3 mg/mL, respectively. The isometric tension was measured by a Grass FT03 force–displacement transducer attached to a Grass 7D polygraph. The responses are expressed as a percentage of the initial contraction achieved with phenylephrine.
Preliminary pharmacological characterization was carried out to determine the mechanism of action involved in the vasorelaxant effect elicited by the P. calyculatus extract. In some experiments, the endothelium was removed by in situ perfusion of the aorta with 1 mL of saline solution containing 0.2% desoxycholate, immediately after the initial flushing with Krebs–Henseleit solution. The absence of endothelium was confirmed by demonstrating that the addition of 1 μM ACh caused <5% relaxation. In order to verify participation of the NO/cyclic guanosine monophosphate (cGMP) pathway in the relaxant effect of the P. calyculatus extract, experiments were performed in the presence of 10 μM ODQ (1H-[1,2,4]oxadiazolo-[4,3,-a]quinoxalin-1-one), an inhibitor of soluble guanylate cyclase (sGC), which is the main effector for NO in arterial smooth muscle cells. In these experiments ODQ was added to the bath 20 min prior to the addition of phenylephrine. ODQ was prepared as a stock solution (10 mM) in dimethyl sulfoxide and stored in aliquots that were further diluted in deionized water.
Results of the experiments are expressed as the mean ± SD from n = 4–6 experiments. Concentration–response curves for the extracts were plotted and fitted to a sigmoidal concentration–response equation using the data analysis and graphics program Prism 4.0 (GraphPad Software, San Diego, CA, USA). Values of the mean effective concentrations (EC50) and maximum vasorelaxant effects (Emax) were obtained from the concentration–response curves.
1,1-Diphenyl-2-picrylhydrazyl (DPPH) scavenging activity
Radical-scavenging activity was determined using the stable radical DPPH, according to the method reported by CitationFukumoto and Mazza (2000). All reactions were carried out in 96-well microplates (Nalge Nunc International, Rochester, NY, USA). A 20 μL aliquot of an 80% methanol solution of the extracts at different concentrations was mixed with 200 μL of 150 μM of DPPH in 80% methanol. The controls contained all the reaction reagents except the extracts or positive control substances (Trolox and butylhydroxytoluene (BHT)). After 30 min incubation at room temperature in the dark, the resultant absorbance was recorded at 520 nm in a VersaMax tunable microplate reader (Molecular Devices Co., Sunnyvale, CA, USA). A randomized block design (three blocks) was used for the experiments; within each block, every treatment was independently applied three times. The percentage of absorbance inhibition was calculated according to the equation: % inhibition = [(absorbance of control – absorbance of sample)/absorbance of control] × 100. The radical-scavenging activities were expressed as the mean inhibitory concentration (IC50) and the Trolox equivalent antioxidant capacity (TEAC). The IC50 was calculated by a nonlinear regression algorithm (Prism 4.0) from the log-concentration inhibition curve, and the TEAC value, an indicator of the antioxidant capacity of the sample relative to Trolox on a molar basis, was estimated from a Trolox calibration curve (CitationVan den Ber et al., 1999).
Ferric reducing antioxidant power (FRAP)
FRAP values were obtained according to the method reported by CitationFiruzi et al. (2005). Briefly, to prepare the FRAP reagent, a mixture of 10 mL of 300 mM acetate buffer (pH = 3.6), 1 mL of 20 mM FeCl3, and 1 mL of 10 mM 2,4,6-tripyridyl-S-triazine (TPTZ) dissolved in 40 mM HCl, was made. Aliquots (25 μL) of each extract (1–2 mg/mL of extract dissolved in MeOH) were placed in quadruplicate in a 96-well microplate (Nalge Nunc International). Then, 175 μL of freshly prepared, warm (37°C) FRAP solution was added to three of the wells, and the same volume of acetate buffer was added to the fourth. The absorbances at 595 nm were monitored by a SpectraMax tunable microplate reader (Molecular Devices Co.) at 0, 4, 10, 30, and 60 min. Blanks and a standard curve of FeSO4 were also included. The results are expressed as μmol FeSO4 per gram of extract. All data are reported as mean ± SD.
Determination of total phenolic and flavonoid contents
The total phenolic content of the extracts was determined according to the Folin–Ciocalteu colorimetric method (CitationDewanto et al., 2002). Appropriate dilutions of the extracts were oxidized with 250 μL of 1 N Folin–Ciocalteu reagent. After 5 min, 1.25 mL of a 20% Na2CO3 solution was added to neutralize for 2 h. The absorbance was measured against a prepared blank at 760 nm. Results are expressed as milligrams of gallic acid equivalents per gram of extract. The total flavonoid content was determined by the method described by CitationLiu et al. (2002). In this test, appropriate dilutions of the extracts were mixed with 75 μL of a 5% NaNO2 solution. After 6 min, 150 μL of a 10% AlCl3·6H2O solution was added, and the mixture was allowed to stand for another 5 min. Then, 0.5 mL of 1 M NaOH was added, followed by distilled water to a total volume of 2.5 mL. The solution was well mixed, and the absorbance was measured against a prepared blank at 510 nm. The results are expressed as milligrams of catechin equivalents per gram of extract. All data are reported as mean ± SD of triplicate analyses.
Identification of flavonoids
Identification of some common flavonoids (hesperitin, quercetin, (+)-catechin, kaempferol, and rutin) in the extracts was performed employing high performance liquid chromatography (HPLC). Flavonoids were primarily identified according to their HPLC retention times, and by comparison with authentic samples. HPLC analysis was carried out on a Waters HPLC system (Millipore Corp., Waters Chromatography Division, Milford, MA, USA) equipped with a 600E multisolvent delivery system, a 990 photodiode array detector, and a 486 UV tunable detector set at 270 nm. A reversed phase column (YMC ODS-QA S5 μm, 4.6 mm × 250 mm, 120 Å) was used. The solvents were: A (1% acetic acid in water) and B (acetonitrile). The flow rate was 0.8 mL/min. The solvent gradient was as follows: 0–15 min, from 90% A to 60% A; 15–20 min, 60% A, 40% B; and back to 90% A.
Results
The results of the pharmacological evaluation, employing the rat aorta model, indicated that all the lyophilized aqueous extracts prepared from the selected species modify the vascular tone. The extracts of A. mexicana, C. murale, C. pentadactylon, D. moldavica, P. calyculatus, P. serotina, and S. edule induced a concentration-dependent relaxation of rat aorta, while the extracts of B. frutescens, M. grandiflora, and S. rostratum stimulated contraction of the aorta.
The most potent stimulatory extract was that of B. frutescens (EC50 = 18 ± 2.4 μg/mL), which also presented the highest maximum contractile response (Emax = 80.6 ± 5.6%). The extracts of M. grandiflora (EC50 = 376.7 ± 48.3 μg/mL) and S. rostratum (EC50 = 1249 ± 77.5 μg/mL) were significantly less potent and they induced maximum contractions of 61.9 ± 4.7% and 44.3 ± 6.6%, respectively.
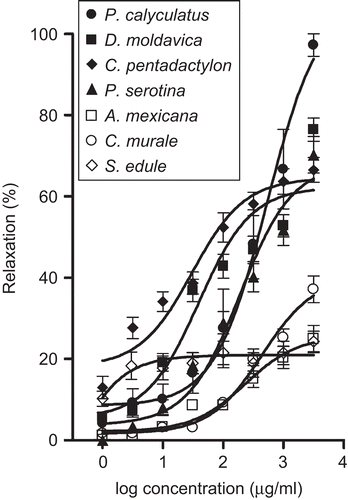
Among the 10 extracts evaluated, seven evoked a relaxation of precontracted aortic rings; however, only the extracts of P. calyculatus, D. moldavica, P. serotina, and C. pentadactylon caused maximum vasorelaxant responses of more than 60%. shows the concentration–response curves for the seven extracts. The values of EC50 and Emax obtained for the extracts are presented in . The extract of P. calyculatus displayed the highest vasorelaxant effect, followed by the extracts of D. moldavica and P. serotina. The extracts of C. murale, A. mexicana, and S. edule induced a maximum vasodilatory effect that was less than 40%. The Emax obtained for ACh, used as a positive control, was 79.8 ± 8.6%.
Table 2. Vasorelaxant effect of ACh and the aqueous extracts prepared from Mexican medicinal plants in rat aortic segments.
Because the extract of P. calyculatus produced the highest vasorelaxant response (Emax = 97.2 ± 2.8%), a preliminary pharmacological characterization of its mechanism of action was performed. The concentration–relaxation curve for the P. calyculatus extract was markedly altered in endothelium-denuded preparations, and the Emax was depressed to 46.9 ± 5.8%, indicating that the vasodilatory effect of the extract was partially endothelium-dependent. To test whether the NO/cGMP pathway was implicated in the P. calyculatus extract-induced relaxation, ODQ was used to inhibit sGC. The ODQ treatment significantly reduced the vasorelaxant effect (Emax = 47.6 ± 4.9%), which resembled that observed in endothelium-denuded aorta.
Since phenolic compounds are among the most abundant plant secondary metabolites that have diverse biological activities, including antioxidant and vasorelaxant effects (CitationGeleijnse et al., 2002; CitationHalliwell et al., 2005; CitationHuxley & Neil, 2003), the total phenolic and flavonoid contents, as well as the antioxidant potential of the extracts, were determined.
shows the contents of total phenolics and flavonoids measured in the extracts that induced vasorelaxation. Total phenolic content ranged from 24.93 to 253.13 mg gallic acid/g extract, and the P. calyculatus extract showed the highest concentration. The flavonoid content also varied over a broad range, from 10.4 to 164.66 mg (+)-catechin equiv./g extract, and was highest in the extract of C. pentadactylon. HPLC analysis of the extracts allowed the identification and quantification of some common flavonoids (). None of the flavonoids used as standards were detected in C. pentadactylon, S. edule, and S. rostratum.
Table 3. Total phenolic and flavonoid contents and antioxidant activity of the plant extracts.
Table 4. HPLC data of flavonoids detected in the plants.
Two in vitro antioxidant capacity assays, DPPH and FRAP, were performed to measure the free-radical-scavenging activity and the total reducing power, respectively, of the extracts. All the extracts were capable of scavenging DPPH radicals, in a concentration-dependent fashion. presents the IC50 and TEAC values. Although there is a broad range of variation within these data, all the extracts were less potent than BHT (IC50 = 56 ± 1.2 µg/mL) and Trolox (IC50 = 71.78 ± 1 µg/mL). The extract of C. pentadactylon exerted the strongest inhibitory effect on DPPH radical generation, while the extract of S. edule was the least active. also shows the FRAP values, which help to assess the total antioxidant activity of the extracts (CitationKatalinic et al., 2006; CitationOzgen et al., 2006). These data indicated significant variation between the capacity of the extracts to reduce Fe3+ to Fe2+ ions, with FRAP values that ranged from 202.90 ± 15.72 to 1150.73 ± 31.70 µmol FeSO4/g, corresponding to the extracts of P. serotina and P. calyculatus, respectively.
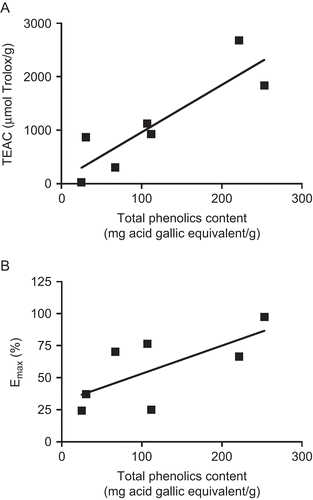
Correlation coefficients between biological activities and phenolic and flavonoid contents were calculated to determine the extent to which these secondary metabolites are responsible for the vasorelaxing and antioxidant effects displayed by the extracts. In addition, the correlation coefficient between maximum vasorelaxant effect and antioxidant capacity was calculated. Regression analysis showed that the total phenolic content significantly correlated with TEAC (r2 = 0.77) () and FRAP (r2 = 0.67). The flavonoid content also correlated with radical-scavenging activity (TEAC: r2 = 0.58), but not with vasorelaxant activity, whereas lower correlations were found between total phenolic content and maximum vasodilatory effect (r2 = 0.48) (), and between antioxidant capacity and maximum vasorelaxant effect (TEAC: r2 = 0.27; FRAP: r2 = 0.49).
Discussion
Endothelial dysfunction, characterized by impaired endothelium-dependent vasodilatation and oxidative stress, is a common pathophysiological feature which develops early in the evolution of hypertension, atherosclerosis, and coronary heart disease (CitationKawashima & Yokohama, 2004; CitationSudano et al., 2006). Increasing of vasorelaxation and reducing of endogenous production of reactive oxygen species have been shown to improve endothelial function and, therefore, to reduce cardiovascular risk (CitationPérez-Vizcaino et al., 2006). The present study demonstrated that aqueous extracts prepared from seven plants, employed in Mexican traditional medicine to treat cardiovascular diseases, promote vascular relaxation and display antioxidant activities. The extract of P. calyculatus elicited a maximum vasodilatory effect greater than that of ACh, a vasorelaxant that causes the release of endothelial nitric oxide (NO) (CitationFurchgott & Zawadzki, 1980), whereas the extracts of C. pentadactylon, D. moldavica, and P. serotina induced maximum relaxations similar to ACh. The aqueous extract of the C. pentadactylon inflorescence and the ethanol extract of P. calyculatus leaves were reported to have vasodilatory actions (CitationPerusquía et al., 1995; CitationRodríguez-Cruz et al., 2003), which is in agreement with the results obtained in this study. In addition, the extracts of C. pentadactylon, P. calyculatus, and D. moldavica exhibited substantial radical-scavenging activity that was 2-, 3.5-, and 4.4-fold less potent than Trolox, respectively. These extracts also exerted significant reducing capacity, which is considered to be proportional to their antioxidant power (CitationHuang et al., 2005). These findings support the hypothesis that the reduction of oxidative stress induced by the plant extracts contributes to their relaxation effect.
Considerable evidence indicates that phenolic compounds, ubiquitous in the plant kingdom, show protective effects on the vessel wall, particularly on endothelial function. Two important mechanisms underlying this protective action are: (1) increased production of endothelial vasorelaxants such as NO, prostanoids, and endothelium-dependent hyperpolarizing factor (EDHF), and (2) scavenging of free radicals that decrease the bioavailability of NO (CitationRamassamy, 2006; CitationSudano et al., 2006; CitationVita, 2005). As expected, the radical-scavenging capacity of the extracts correlated with their polyphenolic and flavonoid contents, indicating that these secondary metabolites are the main antioxidants contained in the extracts. The differential antioxidant activities of the plant extracts observed in the present study are likely to be dependent, not only on the total amount of phenolics, but also on the individual compounds present.
Regression analysis showed that the polyphenolic content correlated with the maximum vasorelaxant effect. This result agrees with previous studies, which have demonstrated that phenolics can cause endothelium-dependent vasodilatation via the release of endothelium-derived vasorelaxants (CitationMullen et al., 2002; CitationSudano et al., 2006). However, the low correlation coefficient suggests that other kinds of secondary metabolites could also be responsible for the vasodilatory properties of the extracts. Additionally, a low correlation coefficient was obtained between the Emax values and antioxidant activity, revealing that other mechanisms apart from the antioxidant activity are involved in the vasorelaxation induced by the plant extracts (CitationSudano et al., 2006). One of these mechanisms is activation of the NO/cGMP pathway, which is involved in the vasodilatory effect produced by the P. calyculatus extract, as evidenced by the results of the experiments performed in the absence of endothelium and in the presence of ODQ. These experiments clearly demonstrated that the P. calyculatus extract induces an endothelium-dependent vasodilatory effect, probably caused by its high content of polyphenols that promote vascular relaxation by inducing the release of NO from vascular endothelium and diminishing NO breakdown by superoxide anions. This observation is consistent with that found by CitationRodríguez-Cruz et al. (2003), who reported that the ethanol extract of P. calyculatus leaves induced an endothelium-dependent vasodilatation that seemed to be mediated by the synthesis of NO. It is well known that NO induces vascular smooth muscle relaxation through activation of sGC, leading to the accumulation of cGMP (CitationMoncada et al., 1991). In this study, the relaxation response to the P. calyculatus extract was reduced when sGC activity was inhibited by ODQ, clearly implicating the NO/cGMP pathway in the vasorelaxant effect of this extract.
The extracts of B. frutescens, M. grandiflora, and S. rostratum induced concentration-dependent contraction of rat aortic rings. This vasoconstrictive effect supports the ethnomedical use of M. grandiflora as a hypertensive agent. Although our data require in vivo confirmation, they suggest that B. frutescens and S. rostratum have no beneficial effects in the treatment of hypertension, as claimed in Mexican traditional medicine. However, our findings showed that these species have alternative potential health benefits in the therapy of other ailments, such as venous insufficiency.
The results derived from this study provide a scientific basis for the traditional uses of the plants in the treatment of cardiovascular diseases. Further investigations are currently in progress in order to identify the metabolites that are responsible for the pharmacological activities of the most active species and to characterize their mechanisms of action.
Acknowledgements
This work was supported by Grant 47433 to one of the authors (M.B.) from the Consejo Nacional de Ciencia y Tecnología (CONACYT). We thank Dr. Dorothy D. Pless for checking the manuscript for correct English usage.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aguilar A, Camacho JR, Chino S, Jácquez P, López ME (1994): Herbario medicinal del Instituto Mexicano del Seguro Social. Mexico, IMSS, pp. 97, 201.
- De la Cruz M (1991): Libellus de Medicinalibus Indorum Herbis. Manuscrito azteca de 1552, según traducción latina de Juan Badiano. Mexico, Fondo de Cultura Económica e Instituto Mexicano del Seguro Social, p. 258.
- Dewanto V, Wu X, Adom K, Lui R (2002): Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50: 3010–3014.
- Díaz JL (1976): Usos de las Plantas Medicinales de México. Monografías Científicas II. Mexico, Instituto Mexicano para el Estudio de las Plantas Medicinales, pp. 17–18.
- Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L (2005): Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta 1721: 174–184.
- Fukumoto LR, Mazza G (2000): Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48: 3597–3604.
- Furchgott RF, Zawadzki JV (1980): The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376.
- Geleijnse JM, Launer LJ, Van der Kuip DAM, Horman A, Witterman JCM (2002): Inverse association of tea and flavonoid intakes with incidence of myocardial infarction: The Rotterdam Study. Am J Clin Nutr 75: 880–886.
- Halliwell B, Rafter J, Jenner A (2005): Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not? Am J Clin Nutr 81: 268S–276S.
- Hernández F (1959): Historia de las plantas de Nueva España. In: Historia Natural de Nueva España, Vols. I and II. México, Universidad Nacional Autónoma de México, pp. 476, 534.
- Huang D, Ou B, Prior R (2005): The chemistry behind antioxidant capacity assays. J Agric Food Chem 53: 1841–1856.
- Huxley R, Neil HAW (2003): The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur J Clin Nutr 57: 904–908.
- Katalinic V, Milos M, Kulisic T, Jukic M (2006): Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem 94: 550–557.
- Kawashima S, Yokohama M (2004): Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol 24: 998–1005.
- Linares E, Bye R, Flores-Peñafiel B (1990): Tés Curativos de México, Cuadernos 7. México, Instituto de Biología, UNAM, pp. 47, 107.
- Linares E, Flores-Peñafiel B, Bye R (1988): Selección de Plantas Medicinales de México. México, Limusa, pp. 20, 44, 52, 98, 100.
- Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH (2002): Antioxidant and antiproliferative activities of raspberries. J Agric Food Chem 50: 2926–2930.
- Martínez M (1991): Plantas medicinales de México. México, Botas, pp. 421–422, 434.
- Meaney E, Lara-Esqueda A, Ceballos-Reyes G, Asbun J, Vela A, Martínez-Marroquín Y, López V, Meaney A, de la Cabada-Tamez E, Velázquez-Monroy O, Tapia-Conyer R (2007): Cardiovascular risk factors in the urban Mexican population: the FRIMEX study. Public Health 121: 378–384.
- Moncada S, Palmer RM, Higgs EA (1991): Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109–142.
- Mullen W, McGinn J, Lean J, MacLean M, Gardner P, Duthie F, Yokota T, Crozier A (2002): Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J Agric Food Chem 50: 5191–5196.
- Norma Oficial Mexicana (2001): Diario Oficial de la Federación. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio (NOM-062-ZOO-1999).
- Ozgen M, Reese RN, Tulio AZ Jr, Scheerens JC, Miller R (2006): Modified 2,2-azino-bis-3-athylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2-2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J Agric Food Chem 54: 1151–1157.
- Pérez-Vizcaino F, Duarte J, Andriantsitohaina R (2006): Endothelial function and cardiovascular disease: Effects of quercetin and wine polyphenols. Free Radic Res 40: 1054–1065.
- Perusquía M, Mendoza S, Bye R, Linares E, Mata M (1995): Vasoactive effects of aqueous extracts from five Mexican medicinal plants on isolated rat aorta. J Ethnopharmacol 46: 63–69.
- Ramassamy C (2006): Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur J Pharmacol S45: 5–64.
- Rodríguez-Cruz M, Pérez-Ordaz L, Serrato-Barajas M, Juárez-Oropeza J, Mascher D, Paredes-Carbajal M (2003): Endothelium-dependent effects of the ethanol extract of the mistletoe Psittacanthus calyculatus on the vasomotor responses of rat aortic rings. J Ethnopharmacol 86: 213–218.
- Sahagún B (1979): Códice Florentino. México, Archivo General de la Nación, Vol. I, p. 353; Vol. II, p. 375.
- Sudano I, Spieker LE, Hermann F, Flammer A, Corti R, Noll G, Lüscher TF (2006): Protection of endothelial function: Targets for nutritional and pharmacological interventions. J Cardiovasc Pharmacol 47: S136–S150.
- Van den Ber R, Haenen GRM, van den Berg H, Bast A (1999): Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem 66: 511–517.
- Velázquez O, Barinagarrementería F, Rubio A, Verdejo J, Méndez M, Violante R, Pavía A, Alvarado-Ruiz R, Lara A (2007): Morbidity and mortality by ischemic heart disease and stroke in Mexico. Arch Cardiol Mex 77: 31–39.
- Vita J, Keaney J (2002): Endothelial function: A barometer for cardiovascular risk? Circulation 106: 640–642.
- Vita J (2005): Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr 81(Suppl.): 292S–297S.