Abstract
The essential oil of fruits of Cuminum cyminum L. (Apiaceae), from India, was analyzed by GC and GC-MS, and its antifungal activity was tested on dermatophytes and phytopathogens, fungi, yeasts and some new Aspergilli. The most abundant components were cumin aldehyde, pinenes, and p-cymene, and a fraction of oxygenate compounds such as alcohol and epoxides. Because of the large amount of the highly volatile components in the cumin extract, we used a modified recent technique to evaluate the antifungal activity only of the volatile parts at doses from 5 to 20 µL of pure essential oil. Antifungal testing showed that Cuminum cyminum is active in general on all fungi but in particular on the dermatophytes, where Trichophyton rubrum was the most inhibited fungus also at the lowest dose of 5 µL. Less sensitive to treatment were the phytopathogens.
Introduction
Natural extracts are in increasing demand from the manufacturers of foods, cosmetics, and pharmaceuticals. The importance of conducting studies on essential oils lies not only in the chemical characterization but also in the possibility of linking the chemical contents with particular functional properties.
This approach acquires further interest with the study of plants from developing countries that are included in programs that aim to reassess their herbal and cultural heritage. The collaboration of our universities with research structures in India translates into a continuous exchange of materials and information. This study is the result of such collaboration.
Cuminum cyminum L. (Apiaceae) is a common plant that occurs in temperate regions Latin America (Chile, Mexico), North Africa (Morocco) and all over Asia including India (Rajasthan, Gujarat and Uttar Pradesh), but its use is very diffused in that country. It is one of the most typical spices of India, especially of the southern part; it is an ancient spice whose history dates back to use in Egypt, it was mentioned in the Bible, used by Greeks, and referenced in the European Middle Ages.
Cumin is one of the most important of Indian condimentary spices and is also widely used in Ayurvedic medicine for the treatment of dyspepsia and jaundice (CitationDhandapani et al., 2002). In indigenous medicine, cumin seeds have long been considered an appetite stimulant and carminative; they are stomachic, astringent, and useful in diarrhea; they improve appetite and taste (CitationMorton, 1976). Noteworthy also is the antimicrobial activity of the essential oil from seeds/fruits (CitationJirovetz et al., 2005).
In this work the chemical composition and the antifungal activity of the essential oil from the fruits (that often are referred to incorrectly as seeds) of Cuminum cyminum were evaluated; in particular, the fungitoxicity of the essential oil volatile components was tested with a new simple and quick technique.
Materials and methods
Plant material
Healthy and mature fruits of Cuminum cyminum were purchased in 2007 from a local Ayurvedic shop of Chhindwara, central India, and authenticated by M. David, Head, Department of Botany, Danielson College, Chhindwara, central India. The fruits were dried at room temperature and then powdered in a grinder. The powder (15 g) was extracted in methanol (Sigma-Aldrich) by Soxhlet apparatus for 48 h and the methanol was evaporated to obtain a semi-solid extract.
Gas chromatography
For the chemical determination, essential oil samples were analyzed by gas chromatography and the relative peak areas for individual constituents averaged. Each sample (1 µL), dissolved in CH2Cl2, was injected in the GC. The relative percentages were determined using a ThermoQuest (Thermo Electron S.p.A., Rodano (MI) Italy) GC-Trace gas chromatograph equipped with a FID detector and a Varian FactorFour (Varian, Inc., Walnut Creek (CA) US) VF-5ms poly-5% phenyl-95%-dimethyl-siloxane bonded phase column (internal diameter 0.25 mm; length 30 m; film thickness 0.15 µm). Operating conditions were as follows: injector temperature 300°C; FID temperature 300°C, carrier gas (helium) flow rate 1 mL/min and split ratio 1:50. Oven temperature was initially 55°C and then raised to 100°C at a rate of 1°C/min, then raised to 250°C at a rate of 5°C/min and finally held at that temperature for 15 min. The percentage composition of the oils was computed by the normalization method from the GC peak areas, without using correction factors.
GC/mass spectrometry analysis
Essential oil constituents were then analyzed by a Varian GC-3800 gas chromatograph equipped with a Varian MS-4000 mass spectrometer using electron impact and connected to the NIST (National Institute of Standards and Technology) library. The constituents of the volatile oils were identified by comparing their GC retention times, KI, and the MS fragmentation pattern with those of other essential oils of known composition, with pure compounds and by matching the MS fragmentation patterns and retention indices with the above mentioned mass spectra libraries and with those in the literature (CitationAdams, 2001). The GC conditions were the same reported for GC analysis and the same column was used. The MS conditions were as follows: ionization voltage, 70 eV; emission current 10 µAmp; scan rate 1 scan/sec; mass range 29-500 Da; trap temperature 150°C, transfer line temperature 300°C. A mixture of aliphatic hydrocarbons (C8-C24) in hexane (Sigma-Aldrich, St. Louis, MO) was injected under the above temperature program to calculate the retention indices using the generalized equation by CitationVan den Dool and Kartz (1963).
Microorganisms
The fungi employed as test organisms were pathogens for humans (dermatophytes) and for plants (phytopathogens), soil saprophytic fungi, and yeasts.
They were: Trichophyton rubrum (Castell.) Sabour, strain number 4321b; Trichophyton mentagrophytes (C.P. Robin) Sabour, strain number160.66a; Trichophyton tonsurans Malmsten, strain number 493.76a; Microsporum gypseum (E. Bodin) Guiart & Grigoraki, strain number 3999b; Nannizzia cajetani Ajello, strain number 3441b (dermatophytes); Botrytis cinerea Pers., strain number 48339c; Fusarium oxysporum Schltdl. strain number 12581c, Pythium ultimum Trow, strain number 58812c; Alternaria spp.; Trichoderma viride strain number 12582c Tul. (phytopathogens); Saccharomyces cerevisiae Meyen ex E.C. Hansen strain number 13057c Rhodotorula glutinis (Fresen.) F.C. Harrison strain number 15125c (yeasts) and Aspergillus amazonicus D. Mares and Aspergillus quitensis D. Mares (saprophytic fungi).
The fungi were obtained from a) the Centraal Bureau voor Schimmelcultures (CBS), Baarn, The Netherlands; b) the Institute of Hygiene and Epidemiology- Mycology (IHME) Brussels, Belgium; c) the American Type Culture Collection (ATCC), Rockville, Maryland, USA; and d) kindly supplied by G. D’Ercole, Plant Pathology Institute, Bologna University, Italy.
The two Aspergilli were isolated and classified by Professor Donatella Mares, Ferrara University, as described in CitationMares et al. (2008).
Antifungal activity
To test the antifungal activity we used a slight modification of the method described by CitationTullio et al. (2006) that enabled us to evaluate the action of volatile components of the essential oil. The fungi were grown on Sabouraud dextrose agar (dermatophytes and yeasts) and potato dextrose agar (phytopathogens and saprophytic fungi) in Petri plates (15 mL/plate) that were inoculated with 6 mm plugs from a culture in the stationary phase; from this moment on the plates were incubated for 24 h at 26° ± 2°C. After this time, sterilized filter paper discs (diameter 6 mm) with different volumes of pure essential oil (5, 10, 15, and 20 µL) were placed in the middle of the lid of each plate. Blanks served as controls. Plates were tightly sealed with parafilm, kept in an inverted position and incubated at 26° ± 2°C. After 7 days the fungal growth was recorded. Growth inhibition was calculated as the percentage of inhibition of radial growth relative to the controls. Three replicates for each experiment were made and the experiments were performed three times.
Results and discussion
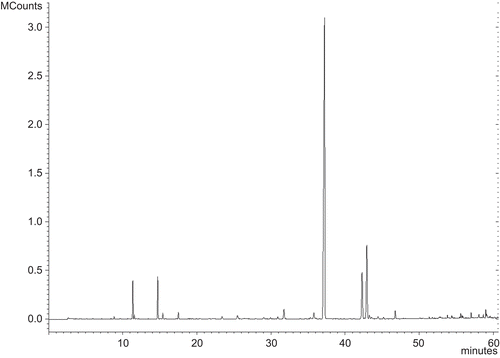
Compared to other cumin oils described in literature (CitationJirovetz et al., 2005), this cumin oil shows a composition with a lower number of components as can be seen in the gas chromatogram ().
The GS/mass spectrometry analysis shows that the most abundant components are aldehydes (81.3%), in particular cumin aldehyde (61.7%), as well as a terpenic fraction not functionalized (pinenes and p-cymene), equal to 9.3%, and a fraction of oxygenate compounds such as alcohol and epoxides, equal to 9.1% ().
Table 1. Percentage composition of the essential oil of fruits from Cuminum cyminum. Compounds in order of elution from a VF-5ms column.
Because phenolic, aldehydic, and alcoholic components of essential oils markedly inhibit filamentous and yeast fungi (CitationBruni et al., 2004), and because of the large amount of these highly volatile components in the cumin extract we used a modified recent technique to evaluate the antifungal activity only of the volatile parts (CitationTullio et al. 2006). In this technique, the inhibition of the fungal growth is due not to a direct contact between the fungal culture and the oil, but to the volatile parts of the oil, so that the microorganism is exposed only to what evaporates at a temperature of 26°C from the paper disc soaked with the oil.
The analysis of data reported in shows that Cuminum cyminum is active in general on all fungi in particular on the dermatophytes. By examining in detail the data of growth inhibition at the different concentrations the cumin oil has a strong activity against dermatophytic fungi, with the exception of T. mentagrophytes and N. cajetani which were less sensitive to treatment at the lower concentrations.
In particular, T. mentagrophytes shows a strange behavior, because at the lower doses it is not affected by the treatment, while at the highest dose of 20 μL reaches 100% inhibition.
Table 2. Percentage inhibition rate after 7 days of treatment with different concentrations of cumin oil.
Very sensitive to treatment are the other three dermatophytes, M. gypseum, T. rubrum, and T. tonsurans, achieving excellent growth inhibitions even at lower doses of 10 and 15 μL. T. rubrum even at the lowest dose of 5 μL reached 100% inhibition.
Generally, the phytopathogens are less sensitive to treatment with respect to dermatophytes, but all show a dose-dependent inhibition that only in one case (P. ultimum) reaches 100%. T. viride shows growth inhibition values lower than that of other phytopathogens.
The two yeasts are both sensitive to treatment: S. cerevisiae shows a dose-dependent inhibition that reached 100% at the highest dose, while R. glutinis showed high values of inhibition at all doses, but never reached 100% inhibition.
Even between the two Aspergilli it is possible to note important differences: while A. quitensis is very sensitive, presenting the highest inhibition already at the dose of 10 μg/mL, A. amazonicus fails to reach 100% inhibition even at the higher dose. This behavior confirms that these two fungi, even though morphologically very similar and present in the same ITS regions (CitationMares et al. 2008), are two different species.
In conclusion, the results of the chemical investigation of the main components of this Indian cumin oil have shown a preponderance of highly volatile components, in particular terpenes and aldehydes. This fact suggested a targeted investigation on antifungal activity that was evaluated by this innovative technique. The obtained results clearly demonstrate that the most volatile components are mainly responsible for the fungitoxicity, confirming what has already been reported in the literature (CitationBruni et al., 2004).
We should also say that this cumin oil has good antifungal activity, particularly against human pathogen fungi such as dermatophytes, and therefore deserves further clinical studies.
Declaration of interest
This work was supported by research grants from MIUR-URST (Ministero dell’Istruzione, dell’Università e della Ricerca – Area Università e Ricerca Scientifica e Tecnologica) of Italy.
References
- Adams RP (2001): Identification of Essential Oil Components by Gas-chromatography/Quadrupole Mass Spectrometry. Carol Stream, IL: Allured Publishing.
- Bruni R, Medici A, Andreotti E, Fantin C, Muzzoli M, Dehesa M, Romagnoli C, Sacchetti G (2004): Chemical composition and biological activities of Ishpingo essential oil, a traditional Ecuadorian spice from Ocotea quixos (Lam.) Kosterm. (Lauraceae) flower calices. Food Chem 85: 415–421.
- Dhandapani S, Subramanian VR, Rajagopal S, Namasivayam N (2002): Hypolipidemic effect of Cuminum cyminum L. on alloxan-induced diabetic rats. Pharmacol Res 46: 251–255.
- Jirovetz L, Buchbauer G, Stoyanova AS, Georgiev EV, Damianova ST (2005): Composition, quality control and antimicrobial activity of the essential oil of cumin (Cuminum cyminum L.) seeds from Bulgaria that had been stored for up to 36 years. Int J Food Sci Technol 40: 305–310.
- Mares D, Andreotti E, Maldonado ME, Pedrini P, Colalongo C, Romagnoli C (2008). Three new species of Aspergillus from Amazonian forest soil (Ecuador). Curr Microbiol 57: 222–229.
- Morton JF (1976). Herbs and Spices. New York: Golden Press, p.160.
- Tullio V, Nostro A, Mandras N, Dugo P, Banche G, Cannatelli MA, Cuffini AM, Alonzo V, Carlone NA (2006). Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J Appl Microbiol 102: 1544–1550.
- Van den Dool H, Kartz PD (1963). A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr 11: 463–471.