Abstract
The antiulcerogenic and antioxidant properties of Matricaria chamomilla L. (Compositae) hydroalcoholic extract (MCE) on ethanol-induced gastric mucosal injury were investigated in rats. After the induction of gastric mucosal injury, all groups were sacrificed; the gastric ulcer index was calculated, and malondialdehyde (MDA) and reduced glutathione (GSH) in whole blood and gastric tissue, and serum ascorbic acid, retinol, and β-carotene levels were measured in all groups. Pretreatment with MCE at some doses significantly reduced gastric lesions. Again, some doses of MCE significantly reduced the MDA, and significantly increased GSH levels in gastric tissue or whole blood. Serum β-carotene and retinol levels were significantly higher in the 200 mg/kg MCE-administered group with respect to control. As a result, MCE clearly has a protective effect against ethanol-induced gastric mucosal lesions, and this effect, at least in part, depends upon the reduction in lipid peroxidation and augmentation in antioxidant activity.
Keywords::
Introduction
Chamomile is one of the most commonly consumed herbal teas. The use of chamomile as a medicinal plant dates back to ancient Greece and Rome. Nowadays it is cultivated in some countries, and its infusion or essential oil forms, due to aromatic and flavoring properties, are widely used in a number of commercial products including herbal teas, foodstuffs, perfumes, soaps, and alcoholic beverages. Matricaria chamomilla L. (Compositae) (MC), locally known as “Papatya”, is widely distributed throughout all regions of Turkey, and naturally found on the road-side and in rural areas. Its infusion (1%) and powder (1–2 g/day) forms are traditionally used for several purposes, including diuresis, sedation, treatment of skin wounds, hemorrhoids, cough, and stomachache (CitationBaytop, 1984; CitationKultur, 2007). Information regarding the beneficial effects, and experimental studies and clinical trials, are presented in recent scientific reports (CitationAchterrath-Tuckermann et al., 1980; CitationAl-Hindawi et al., 1989; CitationAnderson et al., 2000; CitationSavino et al., 2005; CitationCemek et al., 2008). The main active constituents of MC grown in Turkey are resin, coumarin, terpenoids (bisabolol, bisabololoxide, bisabolonoxide, chamazulene), and flavonoids (CitationBaytop, 1984). It contains 0.2–2% volatile oil (CitationBaytop, 1984; CitationMcKay & Blumberg, 2006).
The pathogenesis of peptic ulcer disease is multifactorial, including the chronic use of non-steroidal antiinflammatory drugs, cigarette smoking, and alcohol, and a role of reactive oxygen species (ROS). Some studies have revealed that ROS and lipid peroxidation are implicated in the pathogenesis of ethanol-induced gastric lesions and gastrointestinal damage (CitationBast et al., 1991; CitationCho et al., 1991; CitationLutnicki et al., 1992). It is well known that ethanol causes mucosal hyperemia, which is related to both intramural venoconstriction and submucosal arteriolar dilatation. Lipid peroxide content and oxygen-derived free radicals increase in hypoxic tissue. This condition results in severe changes at the cellular level and causes plasma membrane damage, intracellular calcium accumulation, cell death, exfoliation, and epithelial erosion.
Peptic ulcer is a common disease of the digestive system, and many people suffer from this disease worldwide. Again, peptic ulcer has been known since ancient times, and some medicinal plants are used for the control of ulcer in traditional medicine. Plants with antiulcerogenic activities provide important sources for the development of new drugs in the treatment of peptic ulcer disease. In ulcer phytotherapy, the effects of MC have never been demonstrated in either clinical trials or experimental animal models. Thus, in the present study, we investigated possible antiulcerogenic and antioxidative activities of the hydroalcoholic extract obtained from the aerial parts of MC in an ethanol-induced ulcer model.
Materials and methods
Chemicals
Reduced glutathione (GSH), thiobarbituric acid, phosphate buffer, butylated hydroxytoluene, trichloroacetic acid, ethylenediaminetetraacetic acid (EDTA), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), disodium hydrogen phosphate, phenylendiamine, sodium azide, 2,4-dinitrophenylhydrazine, ethanol, hexane, and sulfanilamide were purchased from Sigma. All other chemicals and reagents used in this study were analytical grade.
Plant material
The aerial parts of MC were collected in May 2005 from Afyonkarahisar (altitude: 1020 m). The plant was identified by Mustafa Kargioglu (Department of Botany of the Science and Arts Faculty, Kocatepe University, Afyonkarahisar, Turkey; Herbarium number: Kala1397). A voucher specimen (B3A) has been kept in our laboratory for future reference.
Extraction and preparation of test samples
Air-dried MC was pulverized with a blender. This plant material (100 g) was extracted on a Soxhlet apparatus using 1 L hydroalcoholic solvent containing 37% ethanol and 63% distilled water. Finally, the solvent was recovered and the extract was lyophilized, weighed (yield: 17.7%), and stored at 4°C, and used to treat the animals as needed. The extract (MCE) was further diluted with distilled water to obtain different doses.
Animals
Sixty-three male Wistar rats with a weight of 150–200 g were used for the experiment. The animal laboratory was windowless with automatic temperature (24 ± 2°C) and lighting (12 h light/dark) controls. The rats were fed with standard laboratory chow and tap water before the experiment. Rats were divided into nine equal groups (n = 7) and housed in different cages. The investigation was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996), and approval was received from our institutional Animal Ethics Committee.
Ulcer study
The antiulcerogenic effect of MCE was investigated using the ethanol-induced ulcer model. Twenty-four hours before the experiment, the rats were fasted and allowed access to water ad libitum. For prevention of coprophagy, rats were placed in cages which had wire-netting floors. On the day of the experiment, group 1 (ethanol group) received 0.5 mL distilled water. Groups 2–6 received 25, 50, 100, 200, and 400 mg/kg MCE, group 7 received 20 mg/kg famotidine (Mustafa Nevzat A.Ş., Turkey), group 8 (intact rats) did not receive any agent, and group 9 received only 100 mg/kg MCE. All drugs were administered by gavage at the same volume (0.5 mL). Following a 60 min period, all the animals in groups 1–7 were given 1 mL of ethanol (80%) by gavage. One hour after the administration of ethanol, rats were injected with a 100 mg/kg dose of ketamine (Pfizer, Turkey); blood samples were taken by cardiac puncture, and stomachs were removed and opened along the greater curvature and washed in physiological saline solution. For measurement of gross gastric mucosal lesions, freshly excised stomachs were laid flat and the mucosal lesions were traced on clear acetate paper. Gross mucosal lesions were recognized as hemorrhage or linear breaks (erosions) with damage to the mucosal surface. The areas of stomach tissue and gross lesions were approximately calculated by planimetry using a simple magnifier. The results were translated to the term “total ulcer area/total gastric area” and these were expressed as an ulcer index (%).
After immediate tracing, the rats’ stomach tissues were homogenized in 10-fold physiological saline solution using a homogenizer (Ultra-Turrax T25, IKA-Werke, 24,000 rpm; Germany). The homogenate was centrifuged at 10,000 g for 1 h to remove debris. The clear upper supernatant was taken, and tissue analysis was carried out on this fraction. All the procedures were performed at +4°C throughout the experiments. Supernatants were stored in polystyrene plastic tubes at −80°C until analysis.
Biochemical study
Fasting blood samples were drawn into heparin-free tubes during routine blood sampling for biochemical analysis. After immediate centrifugation (1000 g for 10 min at 4°C), the serum was stored in polystyrene plastic tubes at −80°C until analysis. Whole blood was collected into heparinized tubes, and whole blood malondialdehyde (MDA) and GSH levels were studied on the same day as admission.
MDA assay
Whole blood MDA (as an important indicator of lipid peroxidation) levels were measured according to the method of CitationJain et al. (1989). The principle of the method is based on spectrophotometric measurement of the color that occurs during the reaction of thiobarbituric acid with MDA. The concentration of thiobarbituric acid reactive substances (TBARS) was calculated from the absorbance coefficient of the malondialdehyde–thiobarbituric acid complex and expressed in nmol/mL.
GSH assay
Estimation of reduced glutathione was done using the method of CitationBeutler et al. (1963) by spectrophotometry. After lysing whole blood and removal of the precipitate, disodium hydrogen phosphate and DTNB solution were added and the color formed was read at 412 nm. The results are expressed in mg/dL.
Ascorbic acid, retinol, and β-carotene analyses
The serum vitamin C (ascorbic acid) level was determined after derivatization with 2,4-dinitrophenylhydrazine (CitationOmaye et al., 1979). The levels of β-carotene at 425 nm and vitamin A (retinol) at 325 nm were detected after the reaction of serum:ethanol:hexane at the ratio of 1:1:3 (CitationSuzuki & Katoh, 1990).
Statistical analysis
All values are expressed as mean ± SD. Statistical analyses of data were performed using a one-way analysis of variance (ANOVA) and Tukey’s post test. A value of p < 0.05 was considered statistically significant.
Results
Effects on acute gastric mucosal lesions induced by ethanol
Macroscopic examination showed that oral administration of 80% ethanol produced multiple mucosal lesions in all rat stomachs. Pretreatment with 50–400 mg/kg MCE significantly decreased the ethanol-induced gastric lesions (decreases of 42.6, 22.8, 58.3, and 50.2%, respectively) with respect to control. This inhibition effect of MCE was highest in the 200 mg/kg group but lower than that of the famotidine group. Famotidine also significantly inhibited the ethanol-induced gastric lesions (the decrease was 70.6%, compared with the control). There was no formation of mucosal lesions in intact and 100 mg/kg MCE-only rats. Ulcer indices (UI) are shown in .
Table 1. Effects of hydroalcoholic extract obtained from aerial parts of Matricaria chamomilla L. (MCE, 25–400 mg/kg) and famotidine (FAM, 20 mg/kg) on ethanol (80%)-induced gastric mucosal injury in rats.
Effects on gastric tissue MDA and GSH levels
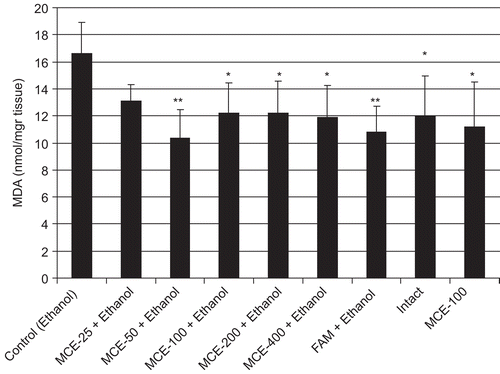
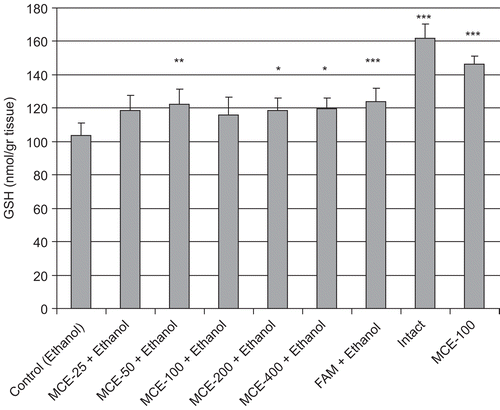
MDA levels of stomach tissue are shown in . The administration of ethanol significantly increased the MDA level in control rat gastric tissue, compared with the intact group (p < 0.05). In contrast, 50–400 mg/kg MCE administration significantly prevented the ethanol-induced gastric tissue MDA rise, compared with the control group, and there was no statistically significant difference between effects of the used MCE doses on MDA level. Additionally, famotidine was found to prevent the rise in MDA level. The GSH level in gastric tissue was significantly lower in the control than in the intact rats. Pretreatment with 50, 200, and 400 mg/kg doses of MCE, or famotidine, significantly prevented the decrease of GSH levels in gastric tissue, with respect to control ().
Effects on whole blood MDA and GSH, and serum vitamin levels
The MDA levels of whole blood are shown in . The administration of ethanol significantly increased the MDA level in whole blood with respect to intact rats (p < 0.001). Pretreatment with MCE decreased the MDA levels, but only the effect of the dose of 50 mg/kg was significant, compared with the control group (p < 0.001). Additionally, famotidine was found to prevent the increase in MDA level (p < 0.01). The GSH level in whole blood was decreased in the ethanol-administered group. In contrast, GSH levels were significantly higher at doses of 50, 100, and 400 mg/kg MCE than that of the control ().
Table 2. Effects of hydroalcoholic extract obtained from aerials part of Matricaria chamomilla L. (MCE, 25–400 mg/kg) and famotidine (FAM, 20 mg/kg) on whole blood MDA and GSH levels (mean ± SD) in rats.
There was no significant difference in the serum ascorbic acid levels (). The administration of ethanol significantly decreased the retinol level in the serum of control rats, with respect to intact (p < 0.01), but there was no significant difference between the serum β-carotene levels of control and intact rats. On the other hand, serum β-carotene and retinol levels were significantly higher in the 200 mg/kg MCE-administered group with respect to control (). Famotidine was found to have no significant effects on serum β-carotene and retinol levels in the rats.
Table 3. Effects of hydroalcoholic extract obtained from aerial parts of Matricaria chamomilla L. (MCE, 25–400 mg/kg) and famotidine (FAM, 20 mg/kg) on serum antioxidant vitamin levels (mean ± SD) in rats.
Discussion
Peptic ulcer is a commonly seen and recurrent benign lesion of the gastrointestinal tract, which occurs at a site where the mucosa is exposed to acid and pepsin. It is frequently treated with inhibitors of gastric acid secretion, which are histamine H2 receptor antagonists, or proton pump inhibitors. The treatment of peptic ulcer disease is still a big challenge, and the development of new antiulcer agents is essential. Many medicinal plants have been shown to be useful against ulcer disease in folk medicine (CitationSchmeda-Hirschmann & Yesilada, 2005). Because of the traditional uses, these plants may be important sources for the development of potential drugs. The present study was aimed to determine the possible protective effect of MCE on the ethanol-induced ulcer model in rats. Therefore, we investigated MCE effects on gastric mucosal lesions as well as some oxidant and antioxidant parameters in rat stomach tissue or blood in order to at least partially explain the mechanism of the MCE antiulcer effect.
Ethanol is one of the commonly used ulcerogenic agents, and when given orally to fasted rats, it produces severe gastric hemorrhagic lesions. Depletion of gastric mucus content, damage to mucosal blood flow, and mucosal cell injury play a role in the pathogenesis of ethanol-induced gastric lesions. Furthermore, ethanol-induced gastric mucosal damage is associated with excessive production of ROS, which leads to increased lipid peroxidation (CitationPihan et al., 1987; CitationSzelenyi & Brune, 1988). The increase in lipid peroxidation and ROS results in marked changes in cellular levels and causes membrane damage, cell death, exfoliation, and epithelial erosion. In the present study, oral administration of ethanol (1 mL/rat, 80%) produced the expected mucosal lesions (hemorrhage, linear breaks, and remarkable hyperemia) in the stomachs of rats. On the other hand, treatment with MCE or famotidine significantly decreased the percentage of lesions ().
Lipid peroxidation has been reported as an important contributor to the loss of cell function under oxidative stress conditions. MDA is an indicator of lipid peroxidation, and its level increases in tissues when they are exposed to oxidative stress. It is reported that MDA production is enhanced after ethanol-induced gastric tissue injury (CitationBirdane et al., 2007; CitationCadirci et al., 2007). In the present study, we observed the high level of MDA in control rats with respect to intact. All doses of MCE, except for 25 mg/kg, significantly decreased the MDA level in gastric tissue compared to the control (). We found that 50 mg/kg is the most effective dose of MCE for decreasing MDA levels, and this prevented MDA formation similarly to famotidine at 20 mg/kg. As for whole blood, a significant MDA-decreasing effect was seen only in 50 mg/kg MCE- and famotidine-administered groups when compared to control ().
The thiol-containing tripeptide GSH is a well-known, important, cellular antioxidant, and has various biological functions in the defense against oxidative stress and xenobiotic toxicity (CitationMeister & Anderson, 1983). In the present study, we found that the mean GSH levels in the stomachs of rats given MCE were increased compared to those of the control group, and this difference in GSH levels in the stomach tissues of rats given 50, 200, and 400 mg/kg MCE was significant. The GSH level in gastric tissue shows an association with the severity of macroscopic damage. Thus, oral administration of ethanol caused a depletion of GSH in the stomachs of rats, but pretreatment with MCE or famotidine prevented this (). Again, some doses of MCE significantly increased the GSH level in whole blood compared to control ().
Non-enzymatic antioxidants such as ascorbic acid, retinol, and β-carotene play an important acute and chronic role in reducing or eliminating the oxidative damage produced by ROS (CitationHalliwell, 1996). In the present study, the mean values of ascorbic acid levels were close, and there was no significant difference between the control and MCE-administered groups. Again, we found that ethanol-induced gastric injury led to a decrease in retinol and β-carotene levels in the untreated group. The mean retinol and β-carotene levels in the sera of rats given MCE were increased, compared to those of the control group, and the difference in retinol and β-carotene levels in the sera of rats given 200 mg/kg MCE was significant. The cause of the increase in antioxidant vitamin levels in the sera of MCE-treated rats might be related to the vitamin content of MCE, or decreased utilization ().
The preliminary phytochemical screening of MC showed the presence of over 120 constituents: amino acids, polysaccharides and fatty acids, volatile oil (α-bisabolol and its oxides, azulenes, farnesene, spathulenol, and spiroethers), flavonoids (apigenin, quercetin, patuletin, luteolin, and their glucosides), and phenolic derivatives (large amounts of ferulic and caffeic acid) are just some of them (CitationBaytop, 1984; CitationMcKay & Blumberg, 2006). These compounds appear to be responsible for the pharmacological actions of MC; they are soluble in hot water, and the amounts obtained from frequent consumption of infusions or teas are not negligible (CitationMcKay & Blumberg, 2006). Chamomile is one of the richest natural sources of apigenin (CitationMcKay & Blumberg, 2006). Previous studies have proved that apigenin is a naturally occurring compound, present in many plants, and possesses chemopreventive, antioxidant, anti-inflammatory, and ulcer healing activity in experimental models (CitationMin et al., 2005; CitationNicholas et al., 2007; CitationPatel et al., 2007). Additionally, α-bisabolol, luteolin, and quercetin are also known to possess antiulcer activity (CitationTorrado et al., 1995; CitationKahraman et al., 2003; CitationMin et al., 2006). The influence of flavonoids on gastric secretion has been studied using pylorus-ligated rats. Intraduodenal administration of flavonoid decreased the volume of gastric juice and increased the gastric pH (CitationWahida et al., 2007). Thus, the presence of the flavonoid content in MCE may partially play a role in ulcer healing due to antisecretory and cytoprotective activities.
In conclusion, the results of the present study show that MCE displays gastroprotective activity, as demonstrated by its significant inhibition of the formation of ulcers induced by ethanol, as well as its ability to decrease lipid peroxidation and enhance antioxidative defense systems. These effects of MCE were not dose-dependent. The antiulcer activity of MCE demonstrated in the present study can provide additional support for the traditional use of this plant in the treatment of stomach ache.
Declaration of interest
This study was supported by grant from the Afyon Kocatepe University research fund (06.FENED.07).
References
- Achterrath-Tuckermann U, Kunde R, Flaskamp E, Isaac O, Thiemer K (1980): Pharmacological investigations with compounds of chamomile; V. Investigations on the spasmolytic effect of compounds of chamomile and Kamillosan on the isolated guinea pig ileum. Planta Med 39: 38–50.
- Al-Hindawi MK, Al-Deen IH, Nabi MH, Ismail MA (1989): Antiinflammatory activity of some Iraqi plants using intact rats. J Ethnopharmacol 26: 163–168.
- Anderson C, Lis-Balchin M, Kirk-Smith M (2000): Evaluation of massage with essential oils on childhood atopic eczema. Phytother Res 14: 452–456.
- Bast A, Haenen GR, Doelman CJ (1991): Oxidants and antioxidants: State of the art. Am J Med 91: 2S–13S.
- Baytop T (1984): Therapy with Medicinal Plants in Turkey (Past and Present), Vol. 3255. Istanbul, Istanbul University Publications, pp. 348–349.
- Birdane FM, Cemek M, Birdane YO, Gülçin I, Buyukokuroglu ME (2007): Beneficial effects of Foeniculum vulgare on ethanol-induced acute gastric mucosal injury in rats. World J Gastroenterol 13: 607–611.
- Beutler E, Dubon O, Kelly BM (1963): Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882–888.
- Cadirci E, Suleyman H, Aksoy H, Halici Z, Ozgen U, Koc A, Ozturk N (2007): Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. Chem Biol Interact 170: 40–48.
- Cemek M, Kağa S, Şimşek N, Büyükokuroğlu ME, Konuk M (2008): Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J Nat Med 62: 284–293.
- Cho CH, Pfeiffer CJ, Misra HP (1991): Ulcerogenic mechanism of ethanol and the action of sulphanilyl fluoride on the rat stomach in vivo. J Pharm Pharmacol 43: 495–498.
- Halliwell B (1996): Antioxidants in human health and disease. Ann Rev Nutr 16: 3–50.
- Jain SK, McVie R, Duett J, Herbst JJ (1989): Erythrocyte membrane lipid peroxidase and glycolylated hemoglobin in diabetes. Diabetes 38: 1539–1543.
- Kahraman A, Erkasap N, Köken T, Serteser M, Aktepe F, Erkasap S (2003): The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology 183: 133–142.
- Kultur S (2007): Medicinal plants used in Kırklareli Province (Turkey). J Ethnopharmacol 111: 341–364.
- Lutnicki K, Wrobel J, Ledwozyw A, Trebas-Pietras E (1992): The effect of calcium ions on the intensity of peroxidation processes and the severity of ethanol-induced injury to the rat’s gastric mucosa. Arch Vet Polon 32: 125–132.
- McKay DL, Blumberg JB (2006): A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother Res 20: 519–530.
- Meister A, Anderson ME (1983): Glutathione. Annu Rev Biochem 52: 711–760.
- Min YS, Bai KL, Yim SH, Lee YJ, Song HJ, Kim JH, Ham I, Whang WK, Sohn UD (2006): The effect of luteolin-7-O-beta-d-glucuronopyranoside on gastritis and esophagitis in rats. Arch Pharm Res 29: 484–489.
- Min YS, Yim SH, Bai KL, Choi HJ, Jeong JH, Song HJ, Park SY, Ham I, Whang WK, Sohn UD (2005): The effects of apigenin-7-O-beta-d-glucuronopyranoside on reflux oesophagitis and gastritis in rats. Auton Autacoid Pharmacol 25: 85–91.
- Nicholas C, Batra S, Vargo MA, Voss OH, Gavrilin MA Wewers MD, Guttridge DC, Grotewold E, Doseff AI (2007): Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J Immunol 79: 7121–7127.
- Omaye ST, Turnbull JD, Sauberlich HE (1979): Ascorbic acid analysis. II. Determination after derivatisation with 2.2. dinitrophenylhydrazine. Selected methods for determination of ascorbic acid in animal cells tissues and fluids. Methods Enzymol 62: 7–8.
- Patel D, Shukla S, Gupta S (2007): Apigenin and cancer chemoprevention: Progress, potential and promise (review). Int J Oncol 30: 233–245.
- Pihan G, Regillo C, Szabo S (1987): Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal damage in rats. Dig Dis Sci 32: 1395–1401.
- Savino F, Cresi F, Castagno E, Silvestro L, Oggero R (2005): A randomized double-blind placebo-controlled trial of a standardized extract of Matricariae recutita, Foeniculum vulgare and Melissa officinalis (ColiMil) in the treatment of breastfed colicky infants. Phytother Res 19: 335–340.
- Schmeda-Hirschmann G, Yesilada E (2005): Traditional medicine and gastroprotective crude drugs. J Ethnopharmacol 100: 61–66.
- Suzuki I, Katoh N (1990): A simple and cheap method for measuring serum vitamin A in cattle using spectrophototmeter. Jpn J Vet Sci 52: 1281–1283.
- Szelenyi I, Brune K (1988): Possible role of oxygen free radicals in ethanol-induced gastric mucosal damage in rats. Dig Dis Sci 33: 865–871.
- Torrado S, Torrado S, Agis A, Jimenez ME, Cadórniga R (1995): Effect of dissolution profile and (–)-alpha-bisabolol on the gastrotoxicity of acetylsalicylic acid. Pharmazie 50: 141–143.
- Wahida B, Abderrahman B, Nabil C (2007): Antiulcerogenic activity of Zizyphus lotus (L.) extracts. J Ethnopharmacol 112: 228–231.