Abstract
Eugenia jambolana Lam. (Myrtaceae) is widely used in folk medicine as an antidiabetic, but there is a lack of information about its toxicity, especially for the stem bark. The present study evaluated acute oral and repeated-dose toxicity of the stem bark aqueous extract of Eugenia jambolana (EJ) in albino mice and Wistar rats. In the acute toxicity tests, mice received oral doses of EJ extract as 300, 2000, and 5000 mg/kg body weight. Mortality, signs of toxicity, body weight, food consumption, and gross findings were observed for 14 days post-treatment. In repeated toxicity, rats were orally treated with 300, 1000, and 2000 mg/kg body weight, and animals were observed till the 28th day of treatment. At the end of the study period, surviving animals were fasted overnight and anesthetized for blood collection and removal of some vital organs for histopathology. No significant differences were noted in body and organ weights between the control and treated groups from either of the studies. In addition, hematological parameters, e.g., red blood cell count (RBC), hemoglobin concentration (Hb), mean corpuscular hemoglobin concentration (MCHC), platelets (PLT), and white blood cell differential count, biochemical parameters, e.g., blood glucose, creatinine, blood urea nitrogen (BUN), alkaline phosphatase (ALP), bilirubin, total protein, and albumin, and ions, e.g., potassium, sodium, chloride, calcium, and phosphorus, were studied in the repeated-dose toxicity study. In conclusion, these investigations indicate the safety of acute and repeated oral administration of the aqueous extract of EJ stem bark, suggesting therefore that it may be continuously used safely.
Introduction
The alternative medicines are believed as being beneficial and free of side effects. The demand for such medicines is increasing day by day for the management and treatment of various health problems (CitationLeonardo et al., 2000). Despite this rapid growth, there is limited evidence for the effectiveness and toxicity of such medicines; much more needs to be done to validate the ethnopharmacological claims with an evidence base for phytomedicines, botanicals, and all natural folklore-originated medicines. The World Health Organization (WHO) has suggested that locally available and traditionally effective herbal drugs be used as alternatives or substitutes for synthetic drugs. With the upsurge in use of herbal medicines, a thorough scientific and systematic exploration of such medicines is imperative (CitationSofowora, 1982).
Eugenia jambolana Lam. (Myrtaceae), commonly known as “Jamun”, is a large evergreen tree with a cylindrical trunk; the stem bark is pale brown to buff white and slightly rough. Most of the plant parts of Eugenia jambolana (EJ) have been used as alternative therapy for diabetes management (CitationGrover et al., 2002; CitationSharma et al., 2003). The leaves in the forms of decoction, tea, and infusion, the fruits, pulp of the fruit, and seeds are very common ingredients in antidiabetic formulations. However, although a part of the same plant, the stem bark has not been studied and explored for its pharmacological activities and toxicity profile. According to Ayurveda, the stem bark of EJ is acrid, sweet, digestive, astringent to the bowels, anthelmentic, antiasthmatic, and antimicrobial; it can be used in cases of thirst, blood impurities, and elevated blood glucose level (CitationNadkarni, 1954; CitationDjipa et al., 2000).
Betulinic acid, gallic acid, kaempferol, ellagitannins, and a few flavonoid and terperne moieties have been reported from the stem bark of EJ (CitationBhargava et al., 1974).
Taking account of all this information and its importance as an alternative medicine, we carried out a toxicological and pharmacological evaluation of the stem bark, which had not been previously studied, in order to bridge the gap between the toxicological profile and ethnopharmacological use in diabetes mellitus.
Hence, the acute and repeated-dose toxicity screening of the aqueous extract of the stem bark was carried out on albino mice and Wistar rats to predict the safety data related to EJ stem bark as an antidiabetic medicine.
Material and methods
Plant material
The stem bark was collected from Baramati (MS), India in the winter season, 2006. The stem bark was authenticated by Dr. P. S. N. Rao of the Botanical Survey of India, Pune (India). A voucher specimen of the same is preserved in the herbarium of the university department.
Preparation of aqueous extract
Extraction of EJ stem bark was carried out using the cold maceration process. Briefly, stem bark (200 g) was moderately powdered and extracted with distilled water (800 mL) for 24 h with frequent shaking. The resulting extract was concentrated by using a vacuum evaporator and preserved in a freeze-dryer. The yield of extract was found to be 18% on a dry weight basis. The extract was prepared freshly by dissolving in double distilled water before administration
Animals
Swiss albino mice and Wistar rats of either sex were used for the experiments. Animals were maintained in the Animal House of the School of Pharmacy and Technology management, NMIMS University, under standard conditions (temperature 25 ± 2°C, relative humidity 75 ± 5%, and 12 h light–dark cycle). During the experiments, animals were provided with a standard rodent pellet diet (Amrut feed) and water ad libitum. All studies were conducted after obtaining prior approval from the institutional animal ethical committee (IAEC) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (CitationNational Institutes of Health, 1985).
Acute toxicity study
Swiss albino mice of both sexes were randomly divided into four groups, each containing six animals (three females and three males, weight: 25 ± 5 g, age: 6–8 weeks). The aqueous extract of stem bark was administered orally at doses of 300, 2000, and 5000 mg/kg of body weight (Organisation for Economic Co-operation and Development (OECD), 2001). Distilled water was administered to the control group. The general behavior of the mice was continuously monitored for 1 h after dosing, periodically during the first 24 h with special attention given during the first 4 h (CitationHilaly et al., 2004), and daily thereafter, for a total of 14 days. A cage-side functional observation battery (FOB) included convulsion, vomiting, diarrhea, paralysis, breathing difficulties, bleeding, irritations, and abnormal posture.
Changes in the normal activity of mice and their body weights were monitored, and the time at which signs of toxicity or death appeared recorded. Signs of toxicity and mortality were observed daily for 13 days, with food and water intake ad libitum. During the study, food consumption was evaluated at 3-day intervals. Body weights of the animals were also recorded at 3-day intervals. All surviving animals were euthanized with diethyl ether at day 14 and various organs including the liver, lungs, heart, spleen, and kidneys were removed, weighed, and carefully examined macroscopically for any abnormal, pathological signs of toxicity.
Repeated toxicity study
Twenty-four Wistar rats were used for the study and randomly assigned into four groups containing six animals in each (weight: 140–180 g; age: 6–8 weeks). Treatment groups were administered orally by gavage once a day for 28 days. The first group of animals, serving as control, received normal saline; the second, third, and fourth groups received the aqueous extract of EJ at doses of 300, 1000, and 2000 mg/kg, respectively.
At the end of the study period, surviving animals were fasted overnight and anesthetized for blood collection from the right ventricle, and some vital organs were examined for histopathology. Blood samples were collected into two different tubes for further accurate analysis of blood: heparinized centrifuge tubes, and dry, non-heparinized centrifuge tubes (CitationAmresha et al., 2008). The heparinized blood was used for a hematological study which included red blood cell count (RBC), hemoglobin concentration (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets (PLT), white blood cell count (WBC) and white blood cell differential count. The non-heparinized blood was allowed to coagulate before being centrifuged and the serum separated. The serum was assayed for blood glucose, creatinine (CRE), blood urea nitrogen (BUN), alkaline phosphatase (ALP), total bilirubin (BIL), total protein (PRO), and albumin (ALB), using readily available kits (Erba, Germany), and ions, i.e. potassium, sodium, chloride, calcium, and phosphorus.
Statistical analysis
All values are expressed as mean ± SD. Statistical comparisons between the data for control and treated groups were performed by means of one-way analysis of variance (ANOVA) followed by Dunnett’s test. A p value less than 0.05 was considered statistically significant.
Results
Acute toxicity
Oral administration of the aqueous extract of EJ up to 5000 mg/kg of body weight did not produce mortality or symptoms of toxicity. According to the OECD guidelines for acute oral toxicity (CitationOrganisation for Economic Co-operation and Development, 2001), an LD50 of 2000 mg/kg and above is categorized as “unclassified”, and hence the drug is found to be safe. So, further dosing to find out the LD50 of EJ stem bark extract was not performed. According to CitationHilaly et al. (2004) in their study of oral administration, the no-observed-adverse-effect (NOAE) dose was found to be 5000 mg/kg of body weight, and the same dose was considered as the maximum tolerated dose (MTD).
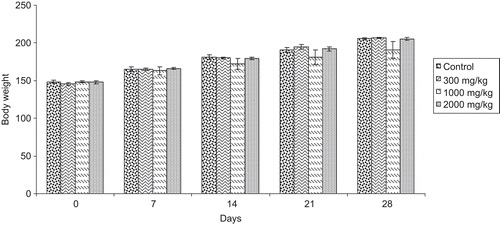
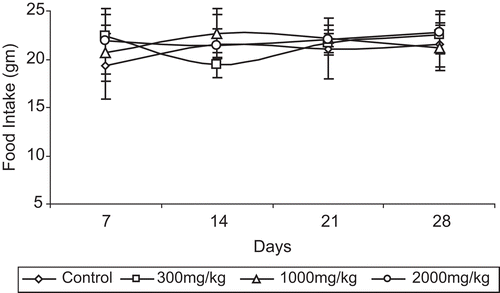
Statistically insignificant differences in the body weights () of both sexes of mice and food consumption trends were noted (), when compared to the control group.
Table 1. Effects of Eugenia jambolana (EJ) aqueous extract on food consumption (g) in acute toxicity after oral administration in mice.
Figure 1. Effects of Eugenia jambolana (EJ) aqueous extract on body weight in acute toxicity study in mice. Data are expressed as mean ± SD, n = 6. No statistical difference between control and EJ extract treated groups.
There were no statistically significant changes observed in weights of some vital organs in male and female mice as compared to the control group in the acute toxicity study.
There was no mortality with any of the above-mentioned doses at the end of 14 days of observation.
Repeated-dose toxicity
No deaths or significant changes in general behavior or other physiological activities were observed at any point in the present study. Clinical signs of neither toxic nor adverse effect were noted throughout the study.
Body weight and food intake
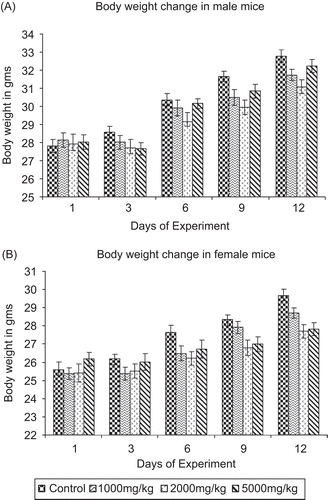
Administration of the extract at doses of 300, 1000, and 2000 mg/kg of body weight for 28 days did not produce any changes from the initial body weight or food intake in the control and treated groups of rats ( and ).
Hematological and biochemical parameters of rats
Hematological analysis () showed no significant changes of RBC, Hb, HT, WBC, and platelets in the treatment group compared to the control group. The leukocyte differential count showed no difference between groups. The biochemical analysis () showed few significant differences in any of the parameters examined in the control or treated groups.
Table 2. Effects of EJ aqueous extract on hematological parameters of rats in repeated toxicity study.
Table 3. Effects of EJ aqueous extract on biochemical parameters of rats in repeated toxicity study.
Organ weights and pathological examinations
There were no significant differences between the control and treated groups in organ weights (). Pathological examination of the tissues on a gross basis indicated that there were no detectable abnormalities. No alterations were seen in microscopic examination of the internal organs; the cellular architecture was not changed in any group.
Table 4. Effects of EJ aqueous extract on weight (g) of internal vital organs of rats in repeated toxicity study.
Discussion
Although there are adequate available data on the phytochemistry, pharmacology, and medicinal uses of the seed, pulp, and leaves of EJ, there are none available with respect to the toxicological profile of the stem bark of EJ.
Usually an acute toxicity study is performed in laboratory animals by giving a high dose, i.e. required to produce death of the animal, with reference to a previous report on toxicity. As such, there was no previous report on the toxicity of EJ except for a few preliminary pharmacological studies on the extract. It did not show any mortality in the present study or in a previous preliminary pharmacological report (CitationMuruganandan et al., 2001).
Therefore, the extract of EJ at dose level 1000–5000 mg/ kg body weight is considered safe, or with negligible toxicity level. Drugs with LD50 1000 and 2000 mg/kg body weight are considered safe according to the report of CitationClarke and Clarke (1977) and OECD guidelines, respectively. The FOB, mean body weights, and consumption of food were not affected by the plant extract, suggesting that the extract does not have any effect on normal behavior or appetite suppression (CitationAmidaa et al., 2007).
At the same time, the results revealed insignificant changes in weight of some vital organs in the experimental groups compared with the control group, justifying the safety of the extract, as organ weight is an important index of the pathophysiological status of humans and animals.
No toxicity signs or deaths were recorded during the 28 consecutive days of treatment by the oral route with EJ at doses of 300, 1000, and 2000 mg/kg. Change in body weight is an indication of an adverse effect of a drug, and is significant when the weight loss is more than 10% from the initial body weight (CitationTeo et al., 2002; CitationRaza et al., 2002; CitationRamesh, 2007). It appeared that the aqueous extract of EJ at the doses used did not produce any marked changes in both male and female rats, as evidenced by the absence of toxic symptoms and no changes in water/food ingestion or weight gain. There was no significant change in total body weight in the present study, which confirms the higher dose as safe.
In the study performed for 28 days, no changes in hematological parameters (i.e. hemoglobin concentration, platelets, red and white blood cells) were revealed between control and treated groups, indicating that the EJ extract was not toxic to the circulating red cells, nor interfered with their production and that of platelets. Hematopoiesis and leukopoiesis were also not affected, even though the hematopoietic system is one of the most sensitive targets for toxic compounds (CitationHarper, 1973) and an important index of physiological and pathological status in man and animals (CitationAdeneye et al., 2006; CitationSteven and Mylecrdfaine, 1994). In the biochemical study, most of the biochemical parameters (e.g. alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholesterol, bilirubin, albumin, and ALP) were also unaffected by the ingested EJ extract, regardless of the dose given. The lack of significant alterations in the levels of ALT, cholesterol, and ALP, as functional indicators of the liver and kidney (CitationHilaly et al., 2004), suggests that higher doses up to 2000 mg/kg of EJ extract did not alter the hepatocytes and kidneys of the rats, and in addition, the normal metabolism of the animals. Although AST, ALP, and BUN demonstrated some significant differences, these could not be fatal because the values were within standard values and/or lower than those of the control group.
All animals survived until the scheduled euthanasia, and no gross pathological alteration was found in the internal organs. Organ weights revealed that the aqueous extract of EJ at the doses used did not produce organ swelling, atrophy, or hypertrophy. Moreover, microscopic evaluation did not find any abnormalities in the group given 2000 mg/kg aqueous extract of EJ compared to the control group.
In conclusion, oral administration of the aqueous extract of EJ was found to be non-toxic to overall behavior and pathophysiological functions of mice and rats. The extract appeared to have no biologically significant toxic effect in both sexes of mice for the entire study. Thus, the present study found EJ extract to be safe up to 5000 mg/kg of body weight in mice.
The present study, therefore, provides satisfactory preclinical evidence of the safety of EJ stem bark extract.
Acknowledgement
The authors are indebted to Dr. D. P. Choudhari, Haffkins Institute, Mumbai for his help in the histopathological studies.
Declaration of interest
Authors declare no conflict of interest.
References
- Adeneye AA, Ajagbonna OP, Adeleke TI, Bello SO (2006): Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J Ethnopharmacol 105: 374–379.
- Amidaa MB, Yemitan OK, Adeyemi OO (2007): Toxicological assessment of the aqueous root extract of Sanseviera liberica Gerome and Labroy (Agavaceae). J Ethnopharmacol 113: 171–175.
- Amresha G, Singh PN, Rao CV (2008): Toxicological screening of traditional medicine Laghupatha (Cissampelos pareira) in experimental animals. J Ethnopharmacol 116: 454–460.
- Bhargava (1974):
- Clarke EGC, Clarke ML (1977): Veterinary Toxicology. London, Cassel & Collier Macmillan, pp. 268–277.
- Djipa CD, Michel M, Quetin-Leclercq J (2000): Antimicrobial activity of bark extracts of Syzygium jambos (L.) Alston (Myrtaceae). J Ethnopharmacol 71: 307–313.
- Grover JK, Yadav S, Vats V (2002): Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol 81: 81–100.
- Harper HA (1973): Review of Physiological Chemistry, 14th ed. Los Altos, CA, Lange Medical Publications.
- Hilaly EJ, Israili ZH, Lyoussi B (2004): Acute and chronic toxicological studies of Ajuga iva in experimental animals. J Ethnopharmacol 91: 43–50.
- Leonardo DCL, Franco A, Gustavo ATL, Luciano MA, Luis FMES, Gabriele PDS, Isabela DMA, José FNN, Israel F, Karla K (2000): Toxicological evaluation by in vitro and in vivo assays of an aqueous extract prepared from Echinodorus macrophyllus leaves. Toxicol Lett 116: 189–198.
- Muruganandan S, Srinivasan K, Chandra S, Tandan SK, Lal J, Raviprakash V (2001): Anti-inflammatory activity of Syzygium cumini bark. Fitoterapia 72: 369–375.
- Nadkarni AK (1954): Indian Materia Medica. Bombay, Popular Prakashan, pp. 1221.
- National Institutes of Health (1985): Guide for the Care and Use of Laboratory Animals. NIH Publication No. 86–23. Bethesda, MD, NIH, Public Health Service.
- Organisation for Economic Co-operation and Development (2001): OECD Guidelines: Acute oral toxicity – acute toxic class method. Paris, OECD, pp. 1–14.
- Ramesh T, Lee K, Lee HW, Kim SJ (2007): Acute oral toxicity study of Asiasari radix extract in mice. Int J Toxicol 26: 247–251.
- Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA (2002): Effect of prolonged vigabatrin treatment on haematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharmaceut 70:135–145.
- Sharma SB, Nasir A, Prabhu KM, Murthy PS, Dev G (2003): Hypoglycemic and hypolipidemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxan-induced diabetic rabbits. J Ethnopharmacol 85: 201–206.
- Sofowora A (1982): Medicinal Plant and Traditional Medicine in Africa, 2nd ed. Ibadan, Nigeria, Wiley, pp. 8–14.
- Steven KR, Mylecrdfaine L (1994): Issues in chronic toxicology. In:Hayes AW, ed., Principles and Methods of Toxicology, 3rd ed. New York: Raven Press, p. 673.
- Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A, Khetani V (2002): A 90-day oral gavage toxicity study of D-methylphenidate and D,L-methylphenidate in Sprague-Dawley rats. Toxicology 179: 183–196.