Abstract
Context: Lansoprazole is a gastric proton-pump inhibitor and has been demonstrated to be effective in the treatment of various peptic diseases. The effects of CYP2C19 activity on the pharmacokinetics of lansoprazole and its active metabolites in Chinese subjects have not previously been evaluated.
Objective: The study aimed to evaluate the effects of CYP2C19 activity in healthy Chinese volunteers.
Materials and methods: Twenty-two healthy volunteers were recruited for an open trial and received a single dose of 30 mg lansoprazole. Using a validated LC-MS/MS method, we measured the plasma concentrations of lansoprazole, 5-hydroxylansoprazole, and lansoprazole sulfone. The genotype of CYP2C19 was identified by polymerase chain reaction (PCR) analysis of single nucleotide polymorphisms (SNPs). Subjects were genotypically classified into the following three groups on the basis of PCR-SNP analysis for CYP2C19: homozygous EM (hmEM) group, heterozygous EM (htEM) group, and PM group. To test differences in pharmacokinetic parameters among the three groups, analysis of variance (ANOVA) after log-transformation of data was used.
Results and conclusion: Our results indicated that there were significant differences (p < 0.001) between the hmEM and PM groups, between the htEM and PM groups, and between the hmEM and htEM groups in Cmax, AUC0–t, and AUC0–inf of lansoprazole and lansoprazole sulfone. There were also significant differences (p < 0.001) between the hmEM and PM groups, and between the htEM and PM groups in Cmax of 5-hydroxylansoprazole.
Introduction
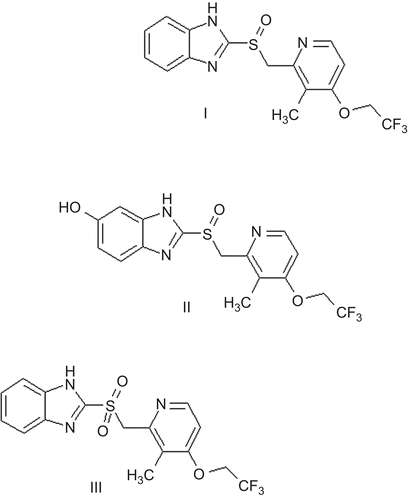
Lansoprazole, 2-[(3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl)methyl] sulfinylbenzimidazole, is a gastric proton-pump inhibitor shown to be effective in the treatment of various peptic diseases, including gastric and duodenal ulcer, reflux esophagitis, and Zollinger–Ellison syndrome (CitationBarradell et al., 1992). Lansoprazole is extensively metabolized in the liver, and the major metabolites present in plasma are 5-hydroxylansoprazole and lansoprazole sulfone (CitationAndersson, 1996; Pearce et al., 1996) (). Formation of the 5-hydroxy metabolite is mainly by cytochrome P450 2C19 (CYP2C19), whereas CYP3A4 is involved in the formation of sulfone (CitationAndersson, 1996; Pearce et al., 1996). CYP2C19, also known as S-mephenytoin 4-hydroxylase, shows genetically determined polymorphism, which is expected to affect the pharmacokinetics of lansoprazole (CitationKim et al., 2002; CitationMiura et al., 2004; CitationLiu et al., 2005). The CYP2C19 gene is located on chromosome 10p, and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis revealed, in addition to the wild-type allele CYP2C19*1, two mutant alleles, CYP2C19*2 and CYP2C19*3, that could be responsible for genetically deficient metabolic activity (CitationDe Morais et al., 1994a, Citation1994b). The metabolic activity of CYP2C19*2 and CYP2C19*3 was almost completely disrupted. However, the heterozygous CYP2C19*1 is sometimes included with homozygous CYP2C19*1 as an extensive metabolizer (EM), without any rational evidence. The combination of CYP2C19*2, *3 and of CYP2C19*2, *2 is considered to give a poor metabolizer (PM). In our study, the CYP2C19 genotype was determined by PCR-single nucleotide polymorphism (SNP) analysis; that is, the homozygous EMs (hmEMs; n = 9; CYP2C19*2 G/G/CYP2C19*3 G/G), the heterozygous EMs (htEMs; n = 8; CYP2C19*2 G/A/CYP2C19*3 G/G, CYP2C19*2 G/G/CYP2C19*3 G/A), and the PMs (n = 5; CYP2C19*2 G/A/CYP2C19*3 G/A, CYP2C19*2 A/A/CYP2C19*3 G/G). The effects of CYP2C19 activity on the pharmacokinetics of lansoprazole in Chinese subjects have been evaluated (CitationHu et al., 2004; CitationQiao et al., 2006), but the effects of CYP2C19 activity on the pharmacokinetics of its active metabolites in Chinese subjects have not been evaluated. The aim of this study was to evaluate the effects of CYP2C19 activity on the pharmacokinetics of lansoprazole and its active metabolites in healthy subjects.
Materials and methods
Study subjects and design
Twenty-two healthy male volunteers, aged 19–24 years, with a body mass index between 19 and 24 kg·m−2, gave informed consent to enter the study. Prior to the study, all subjects underwent physical examinations, safety laboratory tests including blood chemistry, haematology, and routine urine tests, electrocardiogram (ECG), and vital sign assessment. Prior medication (within 2 weeks before the start of the study) was not allowed (with the exception of paracetamol, which was allowed up to 48 h before the start of the study). During the study, no other medications were allowed, except for treatment of adverse events. The study was performed at the Center of Clinical Trials (ZhongShan Hospital, Shanghai, China) and was approved by the local Medical Ethics Committee.
This was an open-label, single-dose pharmacokinetics study with a total of 22 eligible subjects. On the morning of day 1 (treatment period), each eligible subject took a single oral dose of lansoprazole 30 mg after having fasted for at least 10 h at the study center. Each lansoprazole capsule was taken with 200 mL of water. The subjects remained fasting (except for water) during 4 h after dosing. A standardized lunch was served no earlier than 4 h after dosing, and dinner was served no earlier than 10 h after dosing.
Sample collection and analytical methods
During treatment with lansoprazole, pharmacokinetic samples were collected pre-dose and 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, and 24 h after dosing. Blood samples (3 mL) were collected by venous puncture or via an indwelling venous catheter into vacutainers containing heparin natrium. Blood samples were cooled to 4°C and then centrifuged at 1500 g for 10 min at 4°C within 10 min of sampling. The plasma was isolated and transferred into screw-cap polypropylene tubes. Plasma samples were stored at −20°C until shipment to the laboratory for analysis of plasma concentration of lansoprazole and its active metabolites. The liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) method for simultaneous determination of lansoprazole, 5-hydroxylansoprazole, and lansoprazole sulfone in human plasma was validated in terms of linearity, extraction recovery, intra-batch and inter-batch precision and accuracy, specificity, stability, and frozen–unfrozen tests. The lower limits of quantification of lansoprazole, 5-hydroxylansoprazole, and lansoprazole sulfone in plasma were set to 10, 1, and 1 ng/mL, respectively.
CYP2C19 genotyping of subjects
CYP2C19*2, *3 genotyping was determined by PCR of SNPs. Pre-dose blood samples were collected from each of the 22 subjects. Two pairs of primers were designed to amplify two fragments of about 300 bp, including the CYP2C19*2, *3 sites. The primers were designed using the online Primer3 software. The PCR products were purified with shrimp alkali phosphatase and exonuclease I and were then genotyped using the SNaPshot reagent box (ABI Corporation). After purification of the primers, further analysis was performed using capillary electrophoresis with the ABI3130xl gene analyzer (Applied Biosystems, Foster City, USA). The results were analyzed with GeneMapper™ 4.0 software. The CYP2C19*2, *3 genotype results are summarized in .
Table 1. CYP2C19*2, *3 genotype and characteristics of healthy subjects.
Determination of plasma lansoprazole, 5-hydroxylansoprazole, and lansoprazole sulfone pharmacokinetics
Non-compartmental pharmacokinetic (PK) analysis was used to analyze plasma drug concentration–time data. The parameters Cmax (maximum observed concentration) and Tmax (time to reach peak concentration) were obtained directly from experimental observations without interpolation. The terminal slope (Ke) of the concentration–time curve was determined by log-linear regression of at least the last three points. Elimination half-life (t1/2) of the terminal log-linear phase was calculated with the equation 0.693/Ke. Area under the plasma concentration–time curve extrapolated to infinity (AUC0–inf) was determined by summing the areas from time (0) to the time of the last quantifiable concentration by trapezoidal and log-trapezoidal methods (AUC0–t) using the extrapolated area. The extrapolated area was determined by dividing the last detectable concentration by the slope of the terminal log-linear phase. The volume of distribution (V/F) was determined by dividing the administered dose (D) by the area under the plasma concentration–time curve extrapolated to infinity and the terminal slope (Ke) of the concentration–time curve: D/AUC0–inf × Ke. The total body drug clearance (Cl/F) was determined by dividing the administered dose by the area under the plasma concentration–time curve extrapolated to infinity.
Statistical analysis
Pharmacokinetic parameters were summarized for all evaluable subjects by means and standard deviations (SD). To assess differences among the hmEM, htEM, and PM groups in pharmacokinetic parameters of lansoprazole, 5-hydroxylansoprazole, and lansoprazole sulfone, analysis of variance (ANOVA) and nonparametric tests were used.
Results
Pharmacokinetic and statistical analysis of lansoprazole, 5-hydroxylansoprazole, and lansoprazole sulfone for homozygous EM, heterozygous EM, and PM
Non-compartmental pharmacokinetic (PK) analysis was used to analyze plasma drug concentration–time data. The following pharmacokinetics parameters of lansoprazole were determined: the area under the plasma concentration–time curve from 0 to 24 h (AUC0–t) was 4508.81 ± 1241.20 ng.h/mL, 9289.34 ± 3406.73 ng.h/mL, and 36,099.98 ± 10,105.18 ng.h/mL for hmEMs, htEMs, and PMs, respectively; the area under the plasma concentration–time curve from 0 to infinity (AUC0–inf) was 4537.49 ± 1234.38 ng.h/mL, 9434.45 ± 3498.17 ng.h/ mL, and 38,833.30 ± 12,357.60 ng.h/mL for hmEMs, htEMs, and PMs, respectively; the peak plasma concentration (Cmax) was 1830.00 ± 411.28 ng/mL, 2942.50 ± 1018.81 ng/mL, and 4956.00 ± 188.23 ng/mL for hmEMs, htEMs, and PMs, respectively; time to Cmax (Tmax) was 1.67 ± 0.60 h, 1.78 ± 0.49 h, and 2.50 ± 0.61 h for hmEMs, htEMs, and PMs, respectively; elimination half-life (t1/2) was 1.03 ± 0.15 h, 2.17 ± 1.12 h, and 5.80 ± 1.17 h for hmEMs, htEMs, and PMs, respectively; apparent volume of distribution of the central compartment (V/F) was 10.36 ± 2.44 L, 10.11 ± 3.10 L, and 6.71 ± 1.15 L for hmEMs, htEMs, and PMs, respectively; and the apparent clearance (Cl/F) was 7.12 ± 2.20 L/h, 3.61 ± 1.35 L/h, and 0.84 ± 0.27 L/h for hmEMs, htEMs, and PMs, respectively. The following pharmacokinetics parameters of 5-hydroxylansoprazole were determined: AUC0–t was 226.34 ± 82.38 ng.h/mL, 229.43 ± 83.24 ng.h/mL, and 157.27 ± 41.91 ng.h/mL for hmEMs, htEMs, and PMs, respectively; AUC0–inf was 230.55 ± 83.12 ng.h/ mL, 241.79 ± 90.67 ng.h/mL, and 173.40 ± 37.36 ng.h/ mL for hmEMs, htEMs, and PMs, respectively; Cmax was 100.30 ± 38.95 ng/mL, 82.79 ± 38.58 ng/mL, and 28.88 ± 15.64 ng/mL for hmEMs, htEMs, and PMs, respectively; Tmax was 1.56 ± 0.51 h, 1.72 ± 0.56 h, and 2.50 ± 0.61 h for hmEMs, htEMs, and PMs, respectively; t1/2 was 1.95 ± 0.32 h, 2.69 ± 1.12 h, and 5.52 ± 1.84 h for hmEMs, htEMs, and PMs, respectively; V/F was 412.86 ± 166.26 L, 512.50 ± 199.40 L, and 1488.74 ± 686.32 L for hmEMs, htEMs, and PMs, respectively; Cl/F was 144.76 ± 48.49 L/h, 138.27 ± 45.22 L/h, and 179.40 ± 37.28 L/h for hmEMs, htEMs, and PMs, respectively. The following pharmacokinetics parameters of lansoprazole sulfone were determined: AUC0–t was 93.35 ± 43.69 ng.h/mL, 370.49 ± 386.38 ng.h/mL, and 5886.69 ± 1470.72 ng.h/ mL for hmEMs, htEMs, and PMs, respectively; AUC0–inf was 96.11 ± 44.41 ng.h/mL, 377.91 ± 392.17 ng.h/mL, and 6957.79 ± 1768.17 ng.h/mL for hmEMs, htEMs, and PMs, respectively; Cmax was 60.63 ± 25.21 ng/mL, 138.20 ± 52.22 ng/mL, and 414.20 ± 103.11 ng/mL for hmEMs, htEMs, and PMs, respectively; Tmax was 1.67 ± 0.60 h, 1.75 ± 0.52 h, and 4.20 ± 1.64 h for hmEMs, htEMs, and PMs, respectively; t1/2 was 0.72 ± 0.17 h, 1.60 ± 1.08 h, and 7.76 ± 0.79 h for hmEMs, htEMs, and PMs, respectively; V/F was 383.34 ± 176.34 L, 224.71 ± 63.94 L, and 50.71 ± 13.93 L for hmEMs, htEMs, and PMs, respectively; and Cl/F was 389.76 ± 211.76 L/h, 128.64 ± 65.34 L/h, and 4.53 ± 1.08 L/h for hmEMs, htEMs, and PMs, respectively. The results indicated that there were significant differences (p < 0.001) between hmEMs and PMs, between htEMs and PMs, and between hmEMs and htEMs in Cmax, AUC0–t, and AUC0–inf of lansoprazole and lansoprazole sulfone. There were also significant differences (p < 0.001) between hmEMs and PMs, and between htEMs and PMs in Cmax of 5-hydroxylansoprazole. All pharmacokinetics parameters of lansoprazole, 5-hydroxylansoprazole, and lansoprazole sulfone are summarized in . Mean plasma concentration–time curves for lansoprazole, 5-hydroxylansoprazole, and lansoprazole sulfone are shown in .
Figure 2. Mean plasma concentration–time curve of lansoprazole for homozygous EM, heterozygous EM, and PM after a single oral administration of 30 mg lansoprazole.
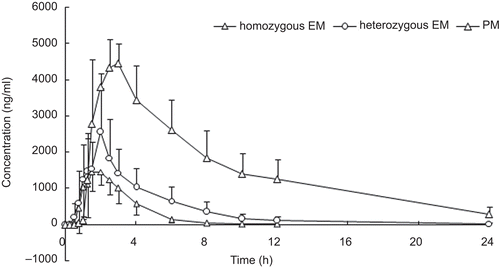
Figure 3. Mean plasma concentration–time curve of 5-hydroxylansoprazole for homozygous EM, heterozygous EM, and PM after a single oral administration of 30 mg lansoprazole.
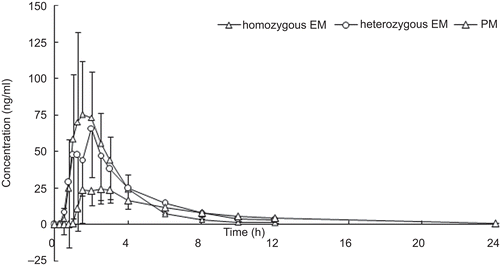
Figure 4. Mean plasma concentration–time curve of lansoprazole sulfone for homozygous EM, heterozygous EM, and PM after a single oral administration of 30 mg lansoprazole.
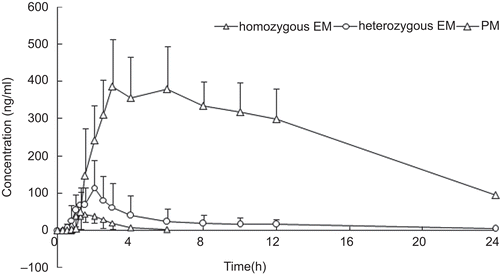
Table 2. Pharmacokinetics results (mean ± SD)of lansoprazole, 5-hydroxylansoprazole, and lansoprazole sulfone for homozygous EM, heterozygous EM, and PM in healthy subjects following single dose of lansoprazole 30 mg.
Safety results
Lansoprazole was safe and well tolerated by all subjects. During the course of the study, no adverse event was reported and no drop-out or death occurred. There were no marked changes in hematology, biochemistry, and urinalysis parameters.
Discussion
Recent developments in pharmacogenomics have suggested that the genetic polymorphisms of drug-metabolizing enzymes, target enzymes, and receptors are responsible for inter-individual variations in efficacy and the incidence of adverse events. The CYP2C19 genotype-related pharmacokinetics of lansoprazole has been reported previously (CitationSakai et al., 2001; CitationUno et al., 2005). These authors reported no significant differences between hmEMs and htEMs in Cmax, AUC0–t, and AUC0–inf of lansoprazole and lansoprazole sulfone. In our study, contrasting results were obtained, and there were significant differences between hmEMs and htEMs. It was demonstrated that the CYP2C19 genotype-dependence of plasma concentrations of lansoprazole could be explained by 5-hydroxylation rather than sulfone formation. The plasma concentrations of 5-hydroxylansoprazole were similar among the three groups, whereas those of lansoprazole sulfone were significantly greater in the PM group compared with the EM groups. To clarify the metabolic process, the AUC values of 5-hydroxylansoprazole and lansoprazole sulfone were corrected by dividing them by the AUC value of lansoprazole. Levels of 5-hydroxylansoprazole were reduced, but sulfone formation was increased in the PM groups, which indicated that it might be a substitute mechanism.
Conclusion
There were significant differences (p < 0.001) between the hmEM and PM groups, between the htEM and PM groups, and between the hmEM and htEM groups in Cmax, AUC0–t, and AUC0–inf of lansoprazole and lansoprazole sulfone. There were also significant differences (p < 0.001) between the hmEM and PM groups, and between the htEM and PM groups in Cmax of 5-hydroxylansoprazole.
Declaration of interest
The authors are grateful to Samoya Kangmeishi Co., Ltd. (Taiwan) and ZhongShan Hospital (Shanghai, China) for financial support.
References
- Andersson T (1996): Pharmacokinetics, metabolism and interactions of acid pump inhibitors. Focus on omeprazole, lansoprazole and pantoprazole. Clin Pharmacokinet 31: 9–28.
- Barradell LB, Faulds D, McTavish D (1992). Lansoprazole: A review of its pharmacodynamic and pharmacokinetic properties and its therapeutic efficacy in acid-related disorders. Drugs 44: 225–250.
- De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA (1994a): Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol 46: 594–598.
- De Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA (1994b): The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem 269: 15419–15422.
- Hu YR, Qiao HL, Kan QC (2004): Pharmacokinetics of lansoprazole in Chinese healthy subjects in relation to CYP2C19 genotypes. Acta Pharmacol Sin 25: 986–990.
- Kim KA, Shon JH, Park JY, Yoon YR, Kim MJ, Yun DH, Kim MK, Cha IJ, Hyun MH, Shin JG (2002): Enantioselective disposition of lansoprazole in extensive and poor metabolizers of CYP2C19. Clin Pharmacol Ther 72: 90–99.
- Liu KH, Kim MJ, Jung WM, Kang W, Cha IJ, Shin JG (2005): Lansoprazole enantiomer activates human liver microsomal CYP2C9 catalytic activity in a stereospecific and substrate-specific manner. Drug Metab Dispos 33: 209–213.
- Miura M, Tada H, Yasui-Furukori N, Uno T, Sugawara K, Tateishi T, Suzuki T (2004): Pharmacokinetic differences between the enantiomers of lansoprazole and its metabolite, 5-hydroxylansoprazole, in relation to CYP2C19 genotypes. Eur J Clin Pharmacol 60: 623–628.
- Pearce RE, Rodrigues AD, Goldstein JA, Parkinson A (1996): Identification of the human P450 enzymes involved in lansoprazole metabolism. J Pharmacol Exp Ther 277: 805–816.
- Qiao HL, Hu YR, Tian X, Jia LJ, Gao N, Zhang LR, Guo YZ (2006): Pharmacokinetics of three proton pump inhibitors in Chinese subjects in relation to the CYP2C19 genotype. Eur J Clin Pharmacol 62: 107–112.
- Sakai T, Aoyama N, Kita T, Sakaeda T, Nishiguchi K, Nishitora Y, Hohda T, Sirasaka D, Tamura T, Tanigawara Y, Kasuga M, Okumura K (2001): CYP2C19 genotype and pharmacokinetics of three proton pump inhibitors in healthy subjects. Pharm Res 18: 721–727.
- Uno T, Yasui-Furukori N, Takahata T, Sugawara K, Tateishi T (2005): Determination of lansoprazole and two of its metabolites by liquid-liquid extraction and automated column-switching high-performance liquid chromatography: Application to measuring CYP2C19 activity. J Chromatogr B 816: 309–314.