Abstract
Alpinia conchigera Griff. (Zingiberaceae), locally known to the Malays as “lengkuas ranting”, is native to Peninsular Malaysia. The Malays traditionally used it to treat infection and rashes, and as a health drink. This study evaluated the analgesic and anti-inflammatory activities of the ethanol extract of A. conchigera rhizomes in mice and rats, respectively. The analgesic activity was elucidated using the acetic acid-induced writhing test, hot plate test, and formalin test, while the anti-inflammatory activity was determined using carrageenan-induced paw edema. The extract (30, 100, and 300 mg/kg) given intraperitoneally (i.p.) exhibited antinociceptive and anti-inflammatory activities in all tests used. The range of percentage of analgesia obtained for all doses of extract in the writhing test was 50–92%, and in the early and late phases of the formalin test was 25–62% and 63–98%, respectively. In addition, naloxone (5 mg/kg) given subcutaneously (s.c.) was found to reverse the extract (300 mg/kg)-induced antinociceptive activity in the writhing, hot plate, and formalin tests. Based on the results obtained, it can be concluded that the ethanol extract of A. conchigera rhizomes possessed a peripheral and central antinociceptive activity that was mediated, in part, via the opioid receptor, as well as anti-inflammatory activity.
Introduction
Malaysia is a tropical country that is rich in natural products, such as herbs, that have been used in the local folk medicine to treat various types of illnesses (e.g. pain and inflammation). The traditional medicinal herbs are still used by most Malaysians extensively as an alternative to modern medicine. One of the many herbs used in Malay traditional medicine is Alpinia conchigera Griff. (Zingiberaceae), locally known as “lengkuas ranting”, which is native to Peninsular Malaysia (CitationIbrahim et al., 2000). A. conchigera is reported to be useful as a food, traditional medicine, spice, condiment, dye, and flavoring (CitationIbrahim et al., 2000).
In terms of its medicinal uses, various parts of the plant (e.g. rhizome, leaves, young shoots, and even the whole plant) have been used to treat illnesses such as muscle pains and sprains, to stop bleeding, etc. (CitationIbrahim et al., 2000). The rhizome extract of A. conchigera, particularly, is traditionally used by the Malays to treat skin fungal infections and rashes, or as a health drink (CitationIbrahim et al., 2000). People in Thailand use its rhizomes to relieve gastrointestinal disorders and in the preparation of Thai food dishes (CitationAthamaprasangsa et al., 1994). CitationAthamaprasangsa et al. (1994) isolated several compounds, including the anti-inflammatory 1,7-disphenyl-3,5-heptanedione, from the rhizomes. In addition, CitationYu et al. (1988) reported that A. conchigera fruits contained anti-inflammatory producing compounds, such as phenylpropanoid derivatives, chavicol acetate, and uegenol acetate. Several species of Alpinia have also been reported to possess different types of pharmacological activities (CitationFonteles & Leal-Cardoso, 1999; CitationTewtrakul et al., 2003; CitationArambewela et al., 2004; CitationAraújo Pinho et al., 2005). The present study investigated the potential antinociceptive and anti-inflammatory properties of the ethanol extract of A. conchigera rhizomes on experimentally induced pain and inflammation, and also aimed to elucidate its mechanisms of action.
Materials and methods
Plant material
Rhizomes of A. conchigera were collected between July and August, 2006 from the Herb Garden, Institute of Bioscience. They were identified by Mr. Shamsul Khamis, a botanist at the Institute of Bioscience (IBS), Universiti Putra Malaysia (UPM), Serdang, Selangor, Malaysia, and a voucher specimen (SK 380/02) has been deposited at the Laboratory of Natural Products, Institute of Bioscience, Universiti Putra Malaysia, Selangor, Malaysia.
Preparation of ethanol extract of Alpinia conchigera rhizomes
Approximately 50 g of the rhizomes of A. conchigera were cleaned, washed, rinsed with distilled water, and dried at 45°C overnight. The dried rhizomes were then ground into powder form and homogenized in 95% ethanol in 1:10, w/v, ratio (CitationEsra et al., 2003). The mixture was then left in an incubator (50°C) for 4 days, followed by the filtration process. The collected supernatant was then evaporated to dryness at 50°C under reduced pressure, and the extract obtained (∼1.34 g; yield of 2.67% (w/w)) was kept at 4°C until use. Prior to use, the dried A. conchigera rhizome ethanol extract (ACRE) was emulsified using 0.1% Tween 80 in normal saline to the doses of 30, 100, and 300 mg/kg body weight for administration to the animals (CitationHosseinzadeh et al., 2000). The doses used were based on a previous study using other species of Alpinia (CitationArambewela et al., 2004).
Chemicals and drugs
Chemicals and drugs used in this research were of analytical grade. Acetic acid and Tween 80 were purchased from Scharlau Chemical Co. (Spain), whereas naloxone hydrochloride, morphine sulfate, acetylsalicylic acid (ASA), pethidine hydrochloride, and carrageenan were purchased from Sigma Chemical Co. (St. Louis, MO, USA). ASA (100 mg/kg), morphine sulfate (5 mg/kg), and pethidine hydrochloride (10 mg/kg) were used as reference drugs.
Animals
Female Balb/C mice (20–30 g) and Sprague-Dawley rats (200–300 g) were obtained from the Animal Unit of the Faculty of Veterinary Medicine, Universiti Putra Malaysia (UPM), Serdang, Selangor, Malaysia. The animals were supplied with a standard commercial diet and water ad libitum and were kept in rooms maintained at 22 ± 2°C, with a 12 h light/dark cycle. The experimental procedures were carried out in strict compliance with the Animal Ethics Committee rules and regulations approved by the Institute (UPM/FPSK/PADS/BR-UUH/00247; 17th April 2006) and the ethical guidelines for investigations of experimental pain in conscious animals (CitationZimmermann, 1983).
Antinociceptive activity
Acetic acid-induced writhing test
The abdominal constriction test was performed as described by CitationCollier et al. (1968) with slight modifications. Nociception was induced by an intraperitoneal (i.p.) injection of 0.6% acetic acid in a volume of 10 mL/kg body weight. Mice were earlier treated i.p. with 0.9% normal saline (control group), 100 mg/kg ASA (reference group), or ACRE (30, 100, and 300 mg/kg), 30 min before acetic acid administration. The abdominal constriction resulting from the injection of acetic acid, consisting of a contraction of the abdominal region together with a stretching of the hind limbs, was counted cumulatively over a period of 30 min, commencing 5 min following acetic acid administration. Antinociceptive activity was indicated by a reduction in the mean number of abdominal constrictions in the test group compared to the control group.
Hot plate test
The hot plate test was used to measure response latency according to the method described by CitationEddy and Leimbach (1953) with slight modification. The temperature of the hot plate (model 7280; Ugo Basile, Italy) was maintained at 50 ± 2°C. The mice were treated i.p. with 0.9% normal saline (control group), 10 mg/kg pethidine (reference group), or ACRE (30, 100, and 300 mg/kg). All test solutions were administered 30 min before the beginning of the experiments. Animals were placed in a perspex cylinder on a heated surface, and the time between placement of the animal on the hot plate and the occurrence of discomfort, indicated by either licking of the paws or jumping off the surface, was recorded as response latency. Mice with baseline latencies of more than 10 s were eliminated from the study, and the cut-off time for hot plate latency was set at 20 s. The latency of discomfort was measured at 0, 30, 60, 120, and 150 min after test solution administration.
Formalin test
The formalin test was carried out according to the method described by CitationHunskaar and Hole (1987) but with slight modifications. Pain was induced by injecting 2.5 µL of 25% formalin in the subplantar region of the left hind paw. Mice were treated i.p. with 0.9% normal saline (control group), 100 mg/kg ASA or 10 mg/kg pethidine (reference groups), or ACRE (30, 100, and 300 mg/kg), 30 min prior to formalin injection. The mice were individually placed in a transparent perspex observation chamber. The amount of time that the animal spent licking the injected paw, considered as an indicator of pain, was recorded during 30 min following the formalin injection. The early phase of nociception, indicating a neurogenic type of pain response, was measured between 0 and 5 min, while the late phase of nociception, indicating an inflammatory type of pain response, was measured 15–30 min after formalin injection (CitationHunskaar & Hole, 1987). The percentage of the inhibition of licking was calculated based on the following ratio (CitationAlexandre et al., 1999):
Analysis of opioid receptor involvement
To elucidate the involvement of the opioid receptor in the antinociceptive activity of the ACRE, separate groups of mice were pre-treated subcutaneously with naloxone (5 mg/kg), a non-selective opioid receptor antagonist, 10 min prior to the extract injection (CitationHosseinzadeh et al., 2000). This was followed 30 min later by subjection of the mice to the abdominal constriction, hot plate, or formalin test as mentioned above.
Anti-inflammatory activity
Carrageenan-induced rat paw edema test
The carrageenan-induced paw edema test was carried out according to the method described by CitationWinter et al. (1962). Paw swelling was induced by subplantar injection of 0.1 mL of 1% sterile λ-carrageenan into the right hind paw of the rats. The animals were treated (i.p.) with 0.9% normal saline (control group), 100 mg/kg ASA (reference group), or ACRE (30, 100, and 300 mg/kg), 30 min before the carrageenan injection. The volume (mL) of the paw was determined with a plethysmometer (Ugo Basile, Italy), before (0 h) and 1, 2, 3, 4, and 5 h after the carrageenan injection (final volume). The paw edema was determined using the following formula (CitationFabricio et al., 2004):
Histological changes associated with carrageenan-induced rat paw edema
After the last paw volume reading was taken for all groups (interval time of 5 h), the animals were immediately sacrificed and the inflamed paws were removed and fixed in 10% formalin. This was later followed by the established tissue histological preparation, and histopathological findings were assessed according to the scoring system described by CitationSperoni et al. (2002).
Phytochemical screening of ACRE
Phytochemical screening of the ACRE was carried out according to the method of CitationIkhiri et al. (1992) to detect the presence of biologically active compound groups, such as alkaloids, saponins, flavonoids, tannins, triterpenes, and steroids.
Determination of the total phenolic and flavonoid contents of ACRE
The total phenolic content of the ACRE was determined using the Folin–Ciocalteu method as described by CitationRagazzi and Veronese (1973). Briefly, 1.0 mL of ACRE was added to 10.0 mL distilled water and mixed with 2.0 mL of Folin–Ciocalteu phenol reagent (Merck-Schuchardt, Hohenbrun, Germany). The mixture was allowed to stand at room temperature for 5 min and subsequently 2.0 mL sodium carbonate was added, which resulted in the formation of a blue complex. The complex was then measured at 680 nm with catechin used as standard for the calibration curve. The phenolic compound content was calibrated using the linear equation based on the calibration curve. The content of phenolic compound was expressed as µg catechin equivalent/g dry weight (dry weight being that of the rhizome of A. conchigera).
This was followed by determination of the total flavonoid content of the ACRE using the AlCl3 method as described by CitationLamaison and Carnet (1990). Briefly, 1.5 mL of ACRE was mixed with an equal volume of 2% AlCl3·6H2O solution (2 g in 100 mL methanol) and vigorously shaken. After 10 min of incubation, the mixture was measured at 367 nm. Catechin was used as standard for the calibration curve. The flavonoid content was calibrated using the linear equation based on the calibration curve. Flavonoid content was expressed as µg catechin equivalent/g dry weight (dry weight being that of the rhizome of A. conchigera).
Statistical analysis
The data are expressed as mean value ± SEM. Statistical analysis involving two groups was performed by means of Student’s t-test, whereas analysis of variance (ANOVA) followed by the Tukey multiple comparison test was used in order to compare more than two groups using SPSS Version 11.0. The histological evaluation data were tested using non-parametric Friedman’s test. From the data obtained, only values of p < 0.05 were considered significant.
Results
Antinociceptive effects of ACRE
The ACRE significantly (p < 0.05) reduced the writhing response induced by 0.6% acetic acid in a dose-dependent manner, as presented in . The percentage of analgesia recorded at the 30, 100, and 300 mg/kg doses of ACRE was 50, 83, and 92%, respectively. The 300 mg/kg dose of ACRE was more effective than 100 mg/kg ASA, which only produced 72% pain inhibition. Pre-treatment with 5 mg/kg naloxone completely blocked (p < 0.05) the antinociceptive effect of 300 mg/kg ACRE.
Table 1. Antinociceptive activity of A. conchigera rhizome ethanol extract (ACRE) assessed by the acetic acid-induced writhing test in mice.
shows the antinociceptive effects of ACRE assessed using the hot plate test. ACRE only at the doses of 100 and 300 mg/kg significantly (p < 0.05) increased the pain thresholds in mice when compared with the control group. The onset of antinociceptive activity at both doses of ACRE was 30 min after their administration and lasted until the end of the experiment. Interestingly, the antinociceptive activity of 300 mg/kg ACRE was of equal strength when compared to 10 mg/kg pethidine, as indicated by the insignificant results at all interval times. Pre-treatment with 5 mg/kg naloxone was also found to cause complete inhibition (p < 0.05) of the 300 mg/kg ACRE antinociceptive activity.
Table 2. Antinociceptive activity of ACRE assessed by the 50°C hot plate test in mice.
ACRE was also found to exhibit a significant (p < 0.05) antinociceptive effect and in a dose-dependent manner when assessed using the formalin test (). The extract, at all doses used, exerted an antinociceptive effect in both the early and late phases of the formalin test. The percentages of analgesia recorded at 30, 100, and 300 mg/kg doses of ACRE at the early and late phases were 25, 47, and 62%, and 63, 96, and 98%, respectively. The 100 mg/kg dose of ASA was only active in the late phase of the test, with 94% analgesia recorded.
Table 3. Antinociceptive activity of ACRE assessed by the formalin test in rats.
Anti-inflammatory effects of ACRE
Treatment of rats with ACRE at all doses used significantly (p < 0.05) decreased the carrageenan-induced paw edema in a dose-dependent manner (). All extracts produced an onset of anti-inflammation within 1 h of their administration that lasted until the end of the experiment (5 h interval time). The highest dose of ACRE (300 mg/kg) produced a remarkably high percentage of edema inhibition, which was in the range of 74–92%, until the end of the experiment. This activity was of equal strength when compared to 100 mg/kg ASA, as indicated by the insignificant results obtained at the respective interval times.
Table 4. Anti-inflammatory activity of ACRE assessed by the carrageenan-induced paw edema test in rats.
Histological sections of the edematous paw skin dermis after treatment with saline or 300 mg/kg ACRE are shown in . The histological section of skin dermis treated with saline showed margination and migration of leukocytes (L), and their accumulation in the focus of injury (). Infiltrations of leukocytes were part of the inflammatory processes occurring after injury. The dermis also showed inflammation, with edematous dermis associated with vasodilatation of the blood vessel. The histological section of skin dermis treated with 100 mg/kg ASA was found to exhibit mild accumulation and infiltration of leukocytes (L) in the dermis.
Figure 1. Photomicrographs showing the histology of paw skin dermis with hematoxylin and eosin staining at 5 h (×40). (A) Skin treated with saline showed margination and migration of leukocytes (L) and their accumulation in the focus of injury. Infiltrations of leukocytes are part of the inflammatory processes occurring after injury. The dermis also showed inflammation with edema associated with vasodilatation of the blood vessel (V). (B) The rat treated with 300 mg/kg ACRE showed scanty or slight leukocytic (L) infiltration. Slight edema (E) can also be seen within the area of injury.
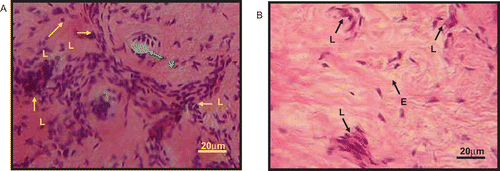
The histological section of the skin dermis after treatment with 30 mg/kg ACRE showed infiltration and accumulation of leukocytes (L), which were not as many as in the saline-treated group, and also localization of edema in the focus of injury. Edema seen in the histological section was noted by an accumulation of excess fluid at the site of injury or spaces within the tissue that were outside the blood vessel. Treatment with 100 mg/kg ACRE was found to cause moderate accumulation of leukocytes in the focus of injury as well as moderate edema. Interestingly, treatment with the 300 mg/kg dose of ACRE was found to cause scanty or slight leukocytic infiltration. Tissue edema could also be seen in the histological section of skin dermis of the rat treated with 300 mg/kg ACRE ().
Phytochemical constituents of ACRE
The phytochemical screening of ACRE demonstrated the presence of a high content of flavonoids and triterpenes and slight presence of saponins, but no tannins, alkaloids, and steroids.
Total phenolic and flavonoid contents of ACRE
The total phenolic content of ACRE, expressed as μmol of catechin equivalent per gram of dry weight, was recorded to be approximately 267.3 ± 6.3 μmol/g dry weight.
The total flavonoid content of ACRE, expressed also as μmol of catechin equivalent per gram of dry weight, was recorded to be approximately 113.4 ± 1.0 μmol/g dry weight.
Discussion
The ACRE was found to possess both antinociceptive and anti-inflammatory activities. The antinociceptive activity of ACRE involved modulation of the peripheral and central mechanisms of pain. The peripheral antinociceptive activity of A. conchigera rhizomes is suggested based on the findings that ACRE inhibited the abdominal constriction test and the second phase of the formalin test, while the central antinociceptive activity of A. conchigera rhizomes is suggested as a result of its ability to prolong the latency of discomfort in the hot plate test and to inhibit the early phase of the formalin test. Interestingly, this study has also revealed the possible involvement of opioid receptors in the modulation of ACRE on peripheral and central antinociceptive activities. In addition, the anti-inflammatory activity of ACRE observed using the carrageenan-induced paw edema test was also supported by the histological finding of the edematous paw treated with ACRE, which showed moderate infiltration of leukocytes to the site of injury when compared to the control group. ACRE also exhibited a characteristic of strong analgesic activity, indicated by its ability to inhibit both the chemical- and thermal-induced nociceptive stimuli (CitationChan et al., 1995), and supported by its ability to inhibit both the early and late phases of the formalin test (CitationAmanlou et al., 2005). Moreover, centrally acting drugs such as opioids inhibit both phases equally, while peripherally acting drugs such as non-steroidal anti-inflammatories (NSAIDs) inhibit only the late phase of the test (CitationAmanlou et al., 2005).
Apart from opioid involvement as described above, the ability of ACRE to exhibit antinociceptive activity could also be due partly to its ability to inhibit the peripheral and central cyclo-oxygenase (COX) action (CitationBallou et al., 2000). Peripheral COX involvement is suggested based on the ability of ACRE to inhibit the writhing response (CitationDeraedt et al., 1980; CitationBerkenkopf & Weichman, 1988) and carrageenan-induced paw edema (CitationGamache et al., 1986). The writhing test has been associated with an increase of prostanoids (prostagalndins PGE2 and PGF2α) released in peritoneal fluids as well as other lipooxygenase (LO) products (CitationVasudevan et al., 2006; CitationChoi et al., 2006), while carrageenan-induced inflammation is more effectively controlled with the arachidonate COX, but not arachidonate LO, inhibitors (CitationGamache et al., 1986). The involvement of central COX in pain response has been reported by CitationPini et al. (1997).
The ability of ACRE to inhibit the release or action of chemical mediators is also proposed to explain the extract’s antinociceptive and anti-inflammatory activities. The writhing response induced by chemical substances is due to sensitization of nociceptors by prostanoids (e.g. prostaglandins) and bradykinins (CitationBerkenkopf & Weichmann, 1988). It is therefore possible that ACRE exhibited its pain-relieving effect probably by inhibiting the action or synthesis of prostaglandins. The formalin test, which consists of two different phases, seems to reflect the involvement of two distinctive types of pain. The earlier phase, resulting from a direct action of formalin on pain receptors, is related to non-inflammatory pain and involves the participation of substance P and bradykinin (CitationShibata et al., 1989). The late phase, on the other hand, involves the release of serotonin, histamine, bradykinin, and prostaglandins (CitationRosland et al., 1990). According to CitationSantos et al. (1994), centrally acting drugs such as morphine inhibit both phases of pain, while peripherally acting drugs such as ASA or indomethacin only inhibit the late phase of the formalin test. Thus, the antinociceptive effect seen in the late phase suggests the ACRE inhibitory effect on inflammatory-mediated pain, which could reflect the extract’s effect on the synthesis, release, or action of prostaglandins, etc. (CitationChoi et al., 2006). The development of edema in the paw of the rat after the administration of carrageenan is characterized by a biphasic process. The initial phase observed during the first hour is attributed to the release of histamine and serotonin, while the second phase of edema is due to the release of prostaglandins (CitationCrunkhorn & Meacock, 1971; CitationVasudevan et al., 2006). CitationSalvemini et al. (1996), on the other hand, described the late phase as being the result of neutrophil infiltration as well as the continuous production of arachidonic metabolites. In addition, the ability of ACRE to reduce carrageenan-induced paw edema indicates its potential as an arachidonate COX inhibitor (CitationGamache et al., 1986). Taking into consideration the histological findings that ACRE reduced paw edema inflammation by reducing leukocyte infiltration (CitationHayashi et al., 1994), it is plausible to suggest, partly, the same mechanism of action for ACRE in the second phase of the formalin test.
Phytochemical screening of ACRE revealed the presence of flavonoids and triterpenes as the major constituents, as well as saponins. Compounds such as flavonoids (CitationKim et al., 2004), triterpenes (CitationBeirith et al., 1999), and saponins (CitationSuh et al., 1996) have been reported to exhibit either antinociceptive or anti-inflammatory activities, and could be linked to the antinociceptive and anti-inflammatory activities of ACRE observed in the present study. Several types of mechanisms could be proposed, based on the phytochemical constituents of the plant, to explain the observed antinociceptive and anti-inflammatory activities. The antinociceptive activity of ACRE could be linked to the findings that flavonoids are potent inhibitors of protein tyrosine kinases (CitationOblak et al., 2000), the protein kinase C pathway (CitationOtuki et al., 2005), and nitric oxide synthase type 2 (NOS-2) (CitationOlszanecki et al., 2002), and have been shown to work via the l-arginine/NO pathway (CitationMeotti et al., 2005). The protein tyrosine kinases, protein kinase C pathway, NOS-2, and l-arginine/NO pathway have been linked with antinociceptive (CitationFerreira et al., 1991; CitationMachelska et al., 1997) as well as anti-inflammatory (CitationKim et al., 2004) activities. Other than that, flavonoids also exhibit inhibitory effects against phospholipase A2 and phospholipase C (CitationMiddleton et al., 2000), and cyclo-oxygenase and/or lipoxygenase pathways (CitationRobak et al., 1998), which play an important role in the nociceptive and inflammatory processes. In addition, flavonoids exert their antinociception via opioid receptor activation, an activity that is not seen with triterpenes or saponins (CitationSuh et al., 1996; CitationRajendran et al., 2000; CitationOtuki et al., 2005). The anti-inflammatory activity of ACRE could be explained by the findings that flavonoids and triterpenes inhibit the nuclear factor-kappaB (NF-κB) (CitationNam, 2006).
In conclusion, ACRE demonstrated antinociceptive and anti-inflammatory activities, with the former activity involving modulation of the opioid receptor system at the peripheral and central levels and the latter activity involving, at least in part, modulation of the infiltration of leukocytes. In addition, the observed activities could be due to the synergistic action of the phytochemical constituents, mainly flavonoids and triterpenes, present in ACRE, and thus confirm the folklore use of the plant for the treatment of pain and inflammatory-related ailments.
Declaration of interest
This research was supported by a Fundamental Research Grant Scheme (FRGS/FASA1-2006/(Sains Perubatan)/UPM/179) from the Ministry of Higher Education, Malaysia.
References
- Alexandre MS, Piuvezam MR, Araujo CC, Thomas G (1999): Studies on the anti-inflammatory and analgesic activity of Curatella amencana L. J Ethnopharmacol 67: 171–177.
- Amanlou M, Dadkhah F, Salehnia A, Farsam H, Dehpour AR (2005): An anti-inflammatory and anti-nociceptive effects of hydrochloric extract of Satureja khuzistanica Jamzad extract. J Pharm Pharm Sci 8: 102–106.
- Arambewela LSR, Arawwawala LDAM, Ratnasooriya WD (2004): Antinociceptive activities of aqueous and ethanol extracts of Alpinia calcarata rhizomes in rats. J Etnopharmacol 95: 311–316.
- Araújo Pinho FVS, Coelho-de-Souza AN, Morais SM, Ferreira Santos C, Leal-Cardoso JH (2005): Antinociceptive effects of the essential oil of Alpinia zerumbet on mice. Phytomedicine 12: 482–486.
- Athamaprasangsa S, Buntrarongroj U, Dampawan P, Ongkavoranan P, Rukachaisirikul V, Sethijinda S, Sornnarintra M, Sriwub P, Taylor WC (1994): A 1,7-diarylheptanoid from Alpinia conchigera. Phytochemistry 37: 871–873.
- Ballou LR, Botting RM, Goorha S, Zhang J, Vane JR (2000): Nociception in cyclooxygenase isozyme-deficient mice. Proc Natl Acad Sci USA 97: 10272–10276.
- Beirith A, Santos ARS, Calixto JB, Hess SC, Messana I, Ferrari F, Yunes RA (1999): Study of the antinociceptive action of the ethanol extract and the triterpene 24-hydroxytormentic acid isolated from the stem bark of Ocotea suaveolens. Planta Med 65: 50–55.
- Berkenkopf JW, Weichman BM (1988): Production of prostacyclin in mice following intraperitoneal injection of acetic acid, phenylbenzoquinone and zymosan: Its role in the writhing response. Prostaglandins 36: 693–709.
- Chan TF, Tsai HY, Tian-Shang W (1995): Anti-inflammatory and analgesic activities from the roots of Angelica pubescens. Planta Med 61: 2–8.
- Choi J-H, Hung B-H, Kang O-H, Choi HJ, Park PS, Cho SH, Kim Y-C, Sohn DH, Park H, Lee JH, Kwon D-Y (2006): The anti-inflammatory and anti-nociceptive effects of ethyl acetate fraction of Cynanchi Paniculati Radix. Biol Pharm Bull 29: 971–975.
- Collier HOJ, Dinneen LC, Jhonson CA, Schneider C (1968): The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol 32: 295–310.
- Crunkhorn P, Meacock SER (1971): Mediators of the inflammation induced in the rat paw by carrageenan. Br J Pharmacol 42: 392–402.
- Deraedt R, Jougney S, Delevalcee F, Falhout M (1980): Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol 51: 17–24.
- Eddy NB, Leimbach D (1953): Synthesis analgesics, II. Dithienylbutenyl and dithienylbutenylamines. J Pharmacol Exp Ther 107: 385–393.
- Esra K, Nurgun H, Erdem JR, Bilge HB (2003): Anti-inflammatory and antinociceptive activity of taxoids and lignans from the heartwood of Taxus baccata L. J Ethnopharmacol 89: 265–270.
- Fabricio HMDM, Jair GGDS, Micheal R, Venusa MNBL, Luzia KAML, Geanne MDAC (2004): Antinociceptive and anti-inflammatory properties of the hydroalcoholic extracts of stems from Equisetum arvense L. in mice. Pharmacol Res 49: 239–243.
- Ferreira SH, Duarte IDG, Lorenzetti BB (1991): The molecular mechanism of action of peripheral morphine analgesia: Stimulation of the cGMP system via nitric oxide release. Eur J Pharmacol 201: 121–122.
- Fonteles MC, Leal-Cardoso JH (1999): Pharmacological effects of essential oils of plants of the Northeast of Brazil. An Acad Bras Cienc 71: 207–213.
- Gamache DA, Povlishock JT, Ellis EF (1986): Carrageenan-induced brain inflammation. Characterization of the model. J Neurosurg 65: 675–685.
- Hayashi K, Nagamatsu T, Ito M, Hattori T, Suzuki Y (1994): Acotoside, a component of Stachys sieboldii MIQ, may be a promising antinephritic agent. Effects of acetoside on crescentic-type anti-GBM nephritis in rats. Jpn J Pharmacol 65: 143–151.
- Hosseinzadeh H, Ramezani M, Salmani GA (2000): Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol 73: 379–385.
- Hunskaar S, Hole K (1987): The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 30: 103–104.
- Ibrahim H, Chooi OH, Hassan R (2000): Ethnobotanical survey of the ginger family in selected Malay villages in Peninsular Malaysia. Malays J Sci 19: 93–99.
- Ikhiri K, Boureima D, Dan-Kouloudo D (1992): Chemical screening of medicinal plants used in the traditional pharmacopoeia of Niger. Int J Pharmacog 30: 251–262.
- Kim HP, Son KH, Chang HW, Kang SS (2004): Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci 96: 229–245.
- Lamaison JLC, Carnet A (1990): Teneurs en principaux flavonoids des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret DC.) en fonction de la vegetation. Pharm Acta Helv 65: 315–320.
- Machelska H, Labuz D, Przewlocki R, Przewlocka B (1997): Inhibition of nitric oxide synthase enhances antinociception mediated by mu, delta and kappa opioid receptors in acute and prolonged pain in the rat spinal cord. J Pharmacol Exp Ther 282: 977–984.
- Meotti FC, Luiz AP, Pizzolatti MG, Kassuya CAL, Calixto JB, Santos ARS (2005): Analysis of the antinociceptive effect of the flavonoid myricitrin. Evidence for a role of the L-arginine-nitric oxide and protein kinase C pathways. J Pharmacol Exp Ther 316: 789–796.
- Middleton E Jr, Kandaswami C, Theoharides TC (2000): The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev 52: 673–751.
- Nam NH (2006): Naturally occurring NF-kappaB inhibitors. Mini Rev Med Chem 6: 945–951.
- Oblak M, Randic M, Solmajer T (2000): Quantitative structure-activity relationship of flavonoid analogues. 3. Inhibition of p56lck protein tyrosine kinase. J Chem Inf Comput Sci 40: 994–1001.
- Olszanecki R, Gêbska A, Kozlovski VI, Gryglewski RJ (2002): Flavonoids and nitric oxide synthase. J Physiol Pharmacol 53: 571–584.
- Otuki MF, Ferreira J, Lima FV, Meyre-Silva C, Malheiros Â, Muller LA, Cani GS, Santos ARS, Yunes RA, Calixto JB (2005): Antinociceptive properties of mixture of α-amyrin and β-amyrin triterpenes: Evidence for participation of protein kinase C and protein kinase A pathways. J Pharmacol Exp Ther 313: 310–318.
- Pini LA, Vitale G, Ottani A, Sandrini M (1997): Naloxone-reversible antinociception by paracetamol in the rat. J Pharmacol Exp Ther 280: 934–940.
- Ragazzi E, Veronese G (1973): Quantitative analysis of phenolics compounds after thin-layer chromatographic separation. J Chromatogr 77: 369–375.
- Rajendran NN, Thirugnanasambandam P, Viswanathan S, Parvathavarthini S, Ramaswamy S (2000): Antinociceptive pattern of flavone and its mechanism as tested by formalin assay. Indian J Exp Biol 38: 182–185.
- Robak J, Shridi F, Wolbis M, Krolikowska M (1998): Screening of the influence of flavonoids on lipoxygenase and cyclooxygenase activity, as well as on nonenzymic lipid oxidation. Pol J Pharmacol Pharm 40: 451–458.
- Rosland JH, Tolsen A, Maehle B, Hole K (1990): The formalin test in mice: Effect of formalin concentration. Pain 42: 235–242.
- Salvemini D, Wang ZQ, Wyatt DM, Bourdon MH, Marino PT, Currie MG (1996): Nitric oxide: A key mediator in the early phase and late phase of carrageenan-induced rat paws inflammation. Br J Pharmacol 118: 829–838.
- Santos AR, Niero R, Filho VC, Yunes RA, Pizzolatti MG, Delle Monache F, Calixto JB (1994): Antinociceptive properties of steroids isolated from Phyllanthus corcovadensis in mice. Planta Med 61: 329–332.
- Shibata M, Ohkubo T, Takahashi H, Inoki R (1989): Modified formalin test: Characteristic biphasic pain response. Pain 38: 347–352.
- Speroni E, Govoni P, Guizzardi S, Renzulli C, Guerra MC (2002): Anti-inflammatory and cicatrizing activity of Echinacea pallida Nutt. root extract. J Ethnopharmacol 79: 265–272.
- Suh HW, Song DK, Son KH, Wie MB, Lee KH, Jung KY, Do JC, Kim YH (1996): Antinociceptive mechanisms of dipsacus saponin C administered intracerebroventricularly in the mouse. Gen Pharmacol 27: 1167–1172.
- Tewtrakul S, Subhadhirasakul S, Kummee S (2003): HIV-1 protease inhibitory effects of medicinal plants used as self medication by AIDS patients. Songklanakrin J Sci Tech 25: 239–43.
- Vasudevan M, Gunnam KK, Parle M (2006): Antinociceptive and anti-inflammatory properties of Daucus carota seeds extract. J Health Sci 52: 598–606.
- Winter CA, Risley EA, Nuss GW (1962): Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111: 544–547.
- Yu J, Fang H, Chen Y, Yao Z (1988): Identification of the chemical components of two Alpinia species. Zhongyao Tongbao 13: 354–356.
- Zimmermann M (1983): Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16: 109–110.