Abstract
Context: Investigating potential central nervous system (CNS) activities of Crataegus monogyna Jacq. (Rosaceae), hawthorn, fruit extracts.
Objective: Evaluating CNS effects and analgesic activities of hawthorn fruit extracts based on the traditional uses of the plant for neurosedative and pain killer actions.
Materials and methods: Effects of hawthorn pulp (HPE) and seed extracts (HSE) at the dose range of 1-1000 mg/kg were examined on anxiety level, spontaneous locomotor activity, motor coordination, and nociceptive perception of mice. Morphine was used as a reference drug.
Results: HPE (100-1000 mg/kg) and HSE (10-1000 mg/kg) significantly decreased not only the exploratory behaviors in hole-board experiments, but also the spontaneous locomotor activities in activity cage tests. The same doses of extracts were found to be ineffective in Rota-Rod tests of mice. In tail-clip, hot-plate, and acetic acid-induced writhing tests, quite potent and dose-dependent analgesic activities were seen at 100-1000 mg/kg doses of HPE and 10-1000 mg/kg doses of HSE. Analgesic effects observed in all analgesia tests were antagonized by naloxone.
Discussion: Significant and dose-dependent decreases in spontaneous locomotor activities and exploratory behaviors of animals suggested CNS depressant activities of both extracts. Complete naloxone antagonism in all applied analgesia tests indicated opioid-related analgesic activities of both extracts.
Conclusion: These findings seem to support the traditional use of this plant to treat stress, nervousness, sleep disorders, and pain control.
Introduction
Crataegus monogyna Jacq. (Rosaceae) also known as Hawthorn, maybush, quick thorn, whitethorn, haw, halves, bread and cheese tree in different countries, is a naturally growing plant in Europe, Asia, and the north of Africa (CitationOzcan et al., 2005; CitationRigelsky & Sweet, 2002). In addition to the consumption of hawthorn berries as food, hawthorn extracts are among the most popular herbal medicinal products in many European countries and the USA due to their cardiovascular effects (CitationPittler et al., 2003). Various parts of the plant (flowers with leaves, fruits) are known for folkloric use especially against cardiovascular disorders (CitationBaytop, 1999; CitationDuke, 2002; CitationMills & Bone, 2000). The effectiveness of hawthorn preparations for the treatments of angina, hypertension, arrhythmias, congestive heart failure, and hyperlipidemia is well documented in a number of preclinical, clinical studies, reviews, and meta-analyses (CitationPittler et al., 2008; CitationRigelsky & Sweet, 2002).
Apart from the cardiovascular disorders, hawthorn fruits have also been used as a cure against stress, nervousness, sleep disorders, heart ache, stomach ache, and sore throat in folk medicine (CitationBaytop, 1999; CitationDuke, 2002; CitationGarcia et al., 1997; CitationMills & Bone, 2000; CitationPietta et al., 1986). In spite of the knowledge about folkloric use of the fruits, there are few reports indicating the anxiolytic and sedative activities of the plant extracts of Crataegus species (CitationBourin et al., 1997; CitationDella Loggia et al., 1981). Furthermore, some constituents of hawthorn fruit extracts have also been demonstrated to exhibit certain effects on the central nervous system (CNS). Amines, flavonoids (vitexine, vitexine-2″-O-rhamnoside, chlorogenic acid, hyperoside, quercetin, isoquercitrin, rutin, etc.), procyanidins, organic acids, tannins, and triterpene derivatives (CitationBaytop, 1999; CitationChang et al., 2002; CitationEuropean Pharmacopoeia, 2005; CitationOrhan et al., 2007) have been reported as the major constituents of hawthorn fruit extracts. Among these compounds, several flavonoids, procyanidins, tannins, chlorogenic acid and various plants containing these constituents have been previously demonstrated to have anxiolytic, sedative and analgesic/antinociceptive activities by many investigators (CitationCalixto et al., 2000; CitationDos Santos et al., 2005, Citation2006; CitationHarborne & Williams, 2000; CitationKang et al., 2000; CitationPérez-Ortega et al., 2008; CitationSoulimani et al., 1997; CitationViana et al., 1997).
To the best of our knowledge, there is no published paper examining CNS activities or antinociceptive/analgesic actions of hawthorn fruits, although the folkloric use of hawthorn fruits has been reported for the cure of stress, nervousness, sleep disorders, and pain (CitationDuke, 2002; CitationMills & Bone, 2000). Therefore, the present study was designed to evaluate the possible effects of hawthorn pulp extract (HPE) and hawthorn seed extract (HSE) on the CNS and pain.
Materials and methods
Plant material and preparation of the extracts
Fresh fruits of Crataegus monogyna plant were collected from the Lake Abant region located in Bolu province of Turkey, in October 2005 and authenticated by Nilgün Öztürk from Anadolu University Faculty of Pharmacy, Department of Pharmacognosy. A voucher specimen (no. ESSE-14438) was deposited at the herbarium of the Laboratory of Botany, Anadolu University, Eskişehir, Turkey. For the extraction, the pulps and seeds of the hawthorn fruit were separated from each other and then dried individually at ambient temperature. The dried materials (15 g) were ground and extracted for 1 h using an ethanol:water mixture (80:20, v/v) in a water bath set at 40°C. The liquid parts were filtered, collected in separate volumetric flasks, and the solid residues were then treated with the ethanol:water solution (80:20, v/v) as described above. This procedure was totally applied for three times to the remaining residues in order to extract the whole chemical content of the plant materials. After extraction, the collected liquid parts were concentrated to dryness under vacuum at 40○C and the final residues were lyophilized. In order to determine extraction yields, the final extracts were weighed and the yields of HPE and HSE were calculated as 37.73% and 11.6%, respectively.
Animals
Adult Swiss albino mice of both sexes weighing 20-30 g were used for the experiments. Male and female mice were distributed into control and experimental groups as homogenously as possible. The animals were housed in a room with controlled temperature (25° ± 1°C) for 12 h light/dark cycle. Temperature, sound and light conditions were not altered during the course of the experiments. All animals were acclimatized to the laboratory environment at least 48 h before the experimental session. Food was withdrawn 12 h before experiments in order to avoid food interference with substance absorption, though water was allowed ad libitum. The experimental protocols were approved by the local ethical committee on animal experimentation, Eskişehir, Turkey.
Administration of drugs and extracts
Morphine sulfate and naloxone used in this study were purchased from Sigma-Aldrich (St. Louis, MO). Acetic acid was supplied from Merck (Darmstadt).
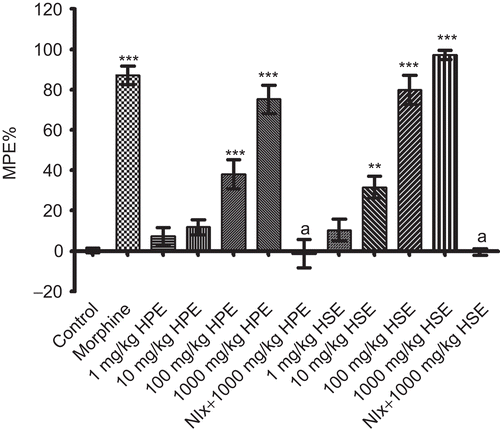
All applications to animals were made by single injections via intraperitoneal (i.p.) route. Test latencies of control solution (0.9% physiological saline), reference drug morphine (10 mg/kg), and the extracts (1, 10, 100, 1000 mg/kg) were recorded 30 min after the administrations. Naloxone (5 mg/kg) was applied 15 min before extract administration to examine a possible involvement of opioid mechanisms in analgesic actions (CitationGomes et al., 2007).
Behavioral tests
Hole-board tests
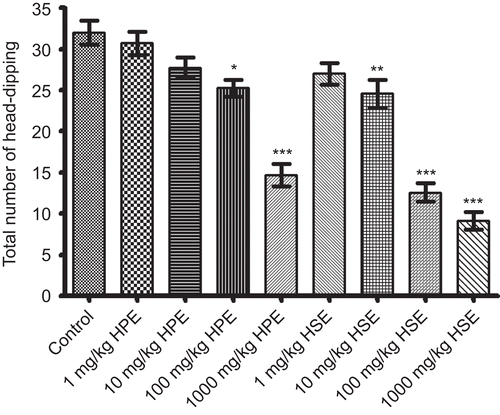
Exploratory behavior of mice (n = 7) was examined by the hole-board test (CitationTakeda et al., 1998). The hole-board apparatus (Ugo Basile, no.6650, Varese, Italy) was composed of a gray Perspex panel (40 × 40 cm) with 16 equidistant holes of 3 cm diameter in the floor. Head-dipping was measured by the infrared cells placed under the holes. The board was positioned 15 cm above the ground. Each animal was individually placed in the center of the board facing away from the observer and allowed to explore the apparatus freely. The total number of head-dipping behavior was recorded for 5 min (CitationFiore et al., 1998; CitationTakeda et al., 1998).
Activity cage measurements
The horizontal and vertical locomotor activities of the mice were recorded by the activity cage apparatus, which contains two pairs of 16 photocells 3 cm and 6 cm above the floor (Ugo Basile, no.7420, Varese, Italy). Interruptions of light beams to the photocells during horizontal and vertical movements of the animals were automatically recorded for 4 min (CitationVotava et al., 2005).
Rota-Rod tests
The effects of extracts on motor coordination levels of mice were examined by the Rota-Rod test. Before the experimental session, three trials were given for three consecutive days on the Rota-Rod apparatus (Ugo Basile, no.47600, Varese, Italy) set at a rate of 16 revolutions per minute. Mice remaining on the rod longer than 180 s were selected for the test. The latency to fall from the rotating mill was recorded for each mouse tested as a criterion of motor coordination (CitationAdzu et al., 2002; CitationAmos et al., 2005).
Analgesia tests
Tail-clip tests
The mechanical antinociceptive activities of the extracts were measured by the tail-clip test in mice (n = 7). A metal artery clamp was applied to the tail of mouse and the time spent before biting the clamp was recorded by a stopwatch (CitationD’Amour & Smith, 1941). A sensitivity test was carried out before the experimental session and animals that did not respond to the clamp within 10 s were discarded from the experiments (CitationAdeyemi et al., 2004). Maximum latency time (cut-off time) for the tail-clip tests was chosen as 10 s to avoid possible tissue damage (CitationOzturk et al., 2002). Analgesia was expressed as a percentage of the maximum possible effect (MPE%), according to the following equation (CitationGabra & Sirois, 2003):
Tail-immersion test
Tail-immersion was conducted as described by CitationAydin et al. (1999). One third of the mouse tail was immersed into a water bath set at a constant temperature of 52.5° ± 0.2°C. The latency between immersion and jerk of the tail was recorded by a stopwatch. A sensitivity test was carried out before the experiments and animals having latencies between the range of 1.5 and 3.5 s were selected for the tests (CitationCoelho et al., 2005). Test latencies were measured with a maximum cut-off time of 10 s to minimize tail tissue damage (CitationGabra & Sirois, 2003). Analgesia was expressed as a percentage of the maximum possible effect (MPE%), calculated by using the equation mentioned above for the tail-clip test.
Hot-plate test
The supraspinal component of antinociceptive action in mice (n = 7) was evaluated by the hot-plate test as described previously (CitationKaplancikli et al., 2009). The mouse was placed in a glass beaker, which was set at a fixed temperature of 55° ± 0.5°C in a water bath and the reaction time of the mouse (latency time for paw licking or jumping) was determined by a stopwatch (Citationde Fátima Arrigoni-Blank et al., 2004). A sensitivity test was carried out before the experiments and only the animals reacting within 15 s were chosen for the tests (CitationShinde et al., 1999). Maximum cut-off time was established as 30 s to prevent tissue damage (Citationde Fátima Arrigoni-Blank et al., 2004). Effects of the extracts on nociception were calculated by converting hot-plate latencies to percentage analgesic activity according to the following equation (CitationAsongalem et al., 2004):
Acetic acid-induced writhing responses
The acetic acid-induced writhing test was applied in mice (n = 7) to investigate the peripheral component of analgesic activity (CitationKoster et al., 1959). Mice were treated with an aqueous solution of acetic acid (0.6% v/v, i.p.) at a dose of 10 mL/kg to induce contractions. Five minutes after the injection of acetic acid solution, the number of abdominal contractions and stretches during the following 10 min was recorded. After pretreatment with extracts or the standard drug, significant reduction in the number of writhings was considered as a positive analgesic response. The percentage protection against writhing was calculated according to following equation (CitationGülçin et al., 2004):
Statistical analyses
The data used in statistical analyses were obtained from seven animals for each of the groups. Experimental data of all tests were analyzed by one-way ANOVA, which was followed by Tukey’s test. Statistical analyses of the experimental data were performed using GraphPad Prism 3.0 software (GraphPad Software, San Diego, CA). The results were expressed as mean ± standard error of mean (SEM). Differences between data sets were considered as significant when p value was less than 0.05.
Results
Behavioral tests
Hole-board tests
As can be seen in , HPE at doses of 100-1000 mg/kg and HSE at doses of 10-1000 mg/kg caused a statistically significant and dose-dependent decrease in the total number of head-dips recorded for 5 min. HPE (1 and 10 mg/kg) and HSE (1 mg/kg) were found to be ineffective on the same experimental parameter in this test.
Activity cage measurements
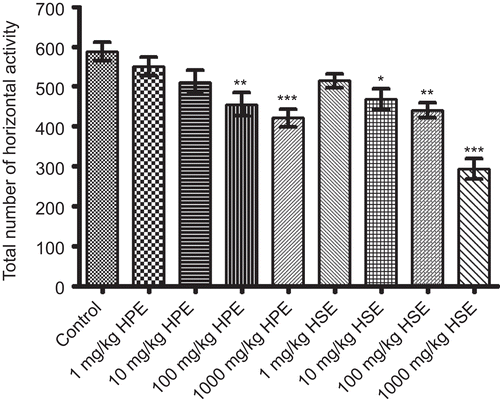
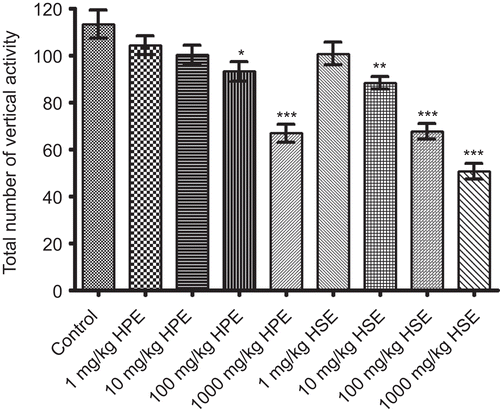
Statistically significant decreases in horizontal and vertical locomotor activities of mice for 4 min were observed following the applications of HPE (100-1000 mg/kg) and HSE (10-1000 mg/kg) when compared to control groups. HPE and HSE at lower doses were not found to cause any significant effect on activity cage parameters of mice ( and ).
Rota-Rod tests
Neither HPE nor HSE were significantly altered the latencies to fall of mice from the rotating mill, when compared to the controls (data not shown).
Analgesia tests
Tail-clip tests
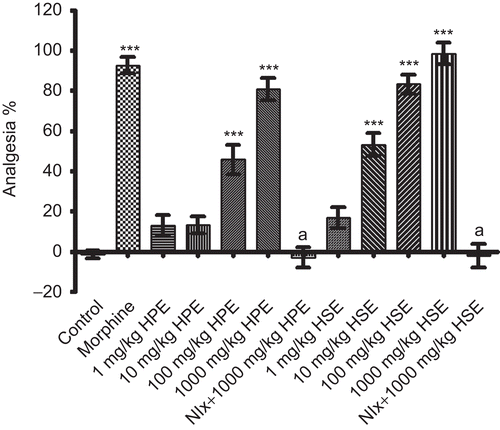
Results obtained from tail-clip tests are indicated in . HPE at doses of 100-1000 mg/kg and HSE at doses of 10-1000 mg/kg exhibited significant and dose-dependent analgesic effects. Doses of 1 mg/kg HSE and 1-10 mg/kg HPE were found to be ineffective. Morphine, which was used as the reference drug, exhibited significant analgesic activity at 10 mg/kg. Analgesic activities of HPE and HSE at the 1000 mg/kg dose disappeared with the pretreatment of naloxone.
Tail-immersion tests
Following the applications of HPE or HSE in tail-immersion tests, no specific change was observed in reaction times of animals compared to control values. Morphine at the dose of 10 mg/kg exhibited significant analgesic activity in this test (data not shown).
Hot-plate tests
illustrates the effects of both extracts on the reaction times of mice against thermal noxious stimuli in hot-plate tests. Dose-dependent and highly significant increases in the reaction times of mice were observed following the administration of HPE at 100 and 1000 mg/kg doses. 1 and 10 mg/kg doses were found to have no effect. Furthermore, HSE at 10-1000 mg/kg doses also potently prolonged the reaction times; only 1 mg/kg dose was ineffective in this test. Morphine showed significant analgesic activity as expected. Pretreatment of naloxone completely antagonized the analgesic activity induced by 1000 mg/kg doses of both HPE and HSE in this test.
Acetic acid-induced writhing responses
As can be seen in , both HPE (100-1000 mg/kg) and HSE (10-1000 mg/kg) reduced the number of acetic acid-induced writhing and stretching. Morphine at the dose of 10 mg/kg exerted a significant protection against the writhing response. Analgesic activities of both extracts at 1000 mg/kg were completely antagonized by naloxone pretreatment ().
Figure 6. Effects of HPE and HSE at doses of 1-1000 mg/kg on response latencies of mice in acetic acid-induced writhing tests. Values are given as mean ± SEM. Significance against control values, *p <0.05, **p <0.01, ***p <0.001; Significance against 1000 mg/kg, ap <0.001, bp <0.01, one-way ANOVA, post-hoc Tukey test, n = 7.
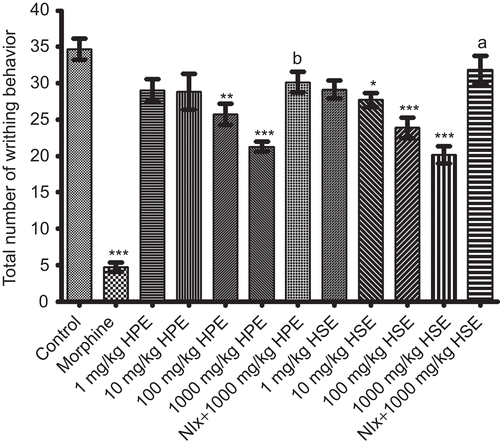
Table 1. Protection percentage values of HPE and HSE extracts in acetic acid-induced writhing tests.
Discussion
The present study was undertaken to investigate the possible effects of Hawthorn fruit extracts (HPE and HSE) on exploratory behavior, spontaneous locomotor activity, motor coordination, and nociception perception of mice by using different experimental models.
In hole-board tests, both extracts significantly and dose-dependently decreased the total number of head-dips, indicating that both extracts depressed the exploratory behavior of mice. Decrease of the vertical activity in activity cage measurements supports the data from the hole-board tests and these findings together suggest that the extracts may have CNS depressant activities. Like vertical activities, number of horizontal locomotor activities was also decreased in activity cage measurements in spite of unchanged motor coordination of mice in Rota-Rod tests. Decrease in the spontaneous locomotor activity without any change in motor coordination also is clear evidence for the neurosedative effects of the HPE and HSE.
In addition to the behavioral tests, analgesia tests were also performed using the same doses of both extracts. Significant and dose-dependent increase in reaction time against noxious stimuli in tail-clip (mechanical stimulus) and hot-plate (thermal stimulus) tests, as well as decrease in the number of writhing and stretching behavior in writhing tests (chemical stimulus) indicate that both HPE and HSE have analgesic actions on all mechanical, thermal and chemical nociceptive neuronal pathways. Hot-plate and tail-clip tests have been reported as a measure of centrally mediated transient pain. As reported previously, the hot-plate test predominantly measures responses organized supraspinally, while the tail-clip test mainly measures spinal reflexes (CitationGabra & Sirois, 2003; CitationWong et al., 1994). As HPE and HSE showed significant analgesic activities in both tail-clip and hot-plate tests, it may be suggested that analgesic activities of the extracts are related to both supraspinal and spinal mechanisms. Analgesic activities of both extracts were reversed completely by the pretreatment of naloxone in these tests, as clear evidence in favor of the involvement of opioid mechanisms in the analgesia. These effects could be due directly to opioid receptor agonistic activities of the constituents in the extracts and/or induction of endogenous opioid peptide release. Furthermore, both extracts protected the animals from writhing in the chemical noxious stimulus-induced writhing test, indicating that peripheral mechanisms may play a role in the analgesic action. Naloxone pretreatments completely reversed the reduction of writhing and stretching behaviors induced by both HPE and HSE at 1000 mg/kg doses in writhing test. Therefore, it may be concluded that the analgesic effects of both extracts observed in the writhing tests also involve opioid mechanisms. It is well known that opioids show analgesic activities in the acetic acid-induced writhing test, but they have relatively lower potencies against chemical noxious stimuli when compared to mechanical and thermal ones (CitationCoelho et al., 2005; CitationMorgan et al., 2006). Similarly, relatively lower analgesic activities of both extracts in the writhing tests compared to those in the tail-clip and hot-plate tests may be due to the lower opioid potency against chemical noxious stimuli.
In order to investigate the possible opioid receptor subtypes contributing to the analgesic action, the tail-immersion test was applied. The tail-immersion test, which was conducted using 52.5°C for thermal algesic stimulus, was reported to discriminate between opioid receptor subtypes (CitationSchmauss & Yaksh, 1984). In spite of significant analgesic effects in hot-plate and tail-clip tests, lack of analgesic activity in the tail-immersion test (at 52.5°C) for any of the applied doses indicates the analgesia induced by both extracts may be related to delta and/or kappa opioid receptor subtypes rather than mu ones (CitationAydin et al., 1999, Citation2003; CitationSchmauss & Yaksh, 1984). Notably, the antinociceptions induced by HPE or HSE even at highest doses were not accompanied with any Straub-tail response, which has been reported to result from the activation of supraspinal mu receptors in mice (Narita et al., Citation1993a). This observation also supports our suggestion that mu receptor subtypes may not be involved in the opioid-related analgesic activities of the HPE and HSE. It has been demonstrated that administration of kappa agonists elicit sedative action in contrast to the stimulant effect of mu and delta-agonists on locomotor activities in mice (CitationIwamoto, 1981; Narita et al., Citation1993b; CitationVonvoigtlander et al., 1983). In the present study, both HPE and HSE were decreased the total number of locomotor activities in the activity cage and reduced the exploratory behavior in the hole-board tests. Therefore, it may be speculated that both HPE and HSE exhibit their analgesic activities related to endogenous opioid system mainly via kappa receptor subtypes. However, the component/components in both extracts may also show their neurosedative effects by different mechanisms, such as acting on GABAergic, glutaminergic, adenosinergic systems etc. The exact mechanisms of action needed to be clarified with further studies.
In the tail-clip, hot-plate and writhing tests HSE showed significant analgesic actions, even at 10 mg/kg dose. When compared to HPE, HSE was more potent in these tests in terms of analgesia percentage. Therefore, the substance(s) responsible for the analgesic activities must be found in higher concentrations in HSE than those in HPE. Some of the flavonoids, procyanidins, organic acids, tannins and triterpene derivatives detected in hawthorn extracts have been reported previously to possess anxiolytic, sedative and analgesic/antinociceptive activities (CitationCalixto et al., 2000; CitationDos Santos et al., 2005, Citation2006; CitationHarborne & Williams, 2000; CitationKang et al., 2000; CitationPérez-Ortega et al., 2008; CitationSoulimani et al., 1997; CitationViana et al., 1997). The precise mechanisms underlying the analgesic action of flavonoids remain unclear, but the major component of this effect seems to be related to an opioid-like action, since there is a significant antagonism by naloxone (CitationKekesi et al., 2003; CitationMaleki-Dizaji et al., 2007; CitationThirugnanasambantham et al., 1988, Citation1990). Therefore, flavonoids, procyanidins, other constituents or their combinations may induce the opioid-mediated analgesic activities as observed in our experiments. However, searching for the responsible component(s) from the pharmacological actions was beyond the scope of this study. Active substances responsible for the pharmacological actions of these extracts will be the subject of new studies in our laboratory.
Conclusion
The results obtained in this study indicate that both HPE and HSE possess not only CNS depressant activities, but also central and peripheral analgesic effects mediated by endogenous opioid system. These findings seem to support the traditional use of this plant to treat stress, nervousness, sleep disorders, and pain control. A relatively high LD50 value (about 6000 mg/kg) reported for hawthorn fruits indicates that its toxicity level is quite low (CitationDuke, 2002). Our study also supports this report, as no animal died by the i.p. application of HPE or HSE, even at the highest dose of 1000 mg/kg. In addition to the potent analgesic activities, low toxicity is an important advantage for the extracts as a novel candidate of analgesic drug.
Declaration of interest
There is no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adeyemi OO, Okpo SO, Okpaka O (2004): The analgesic effect of the methanolic extract of Acanthus montanus. J Ethnopharmacol 90: 45–48.
- Adzu B, Amos S, Dzarma S, Wambebe C, Gamaniel K (2002): Effect of Zizypus spina-christi wild aqueous extract on the central nervous system in mice. J Ethnopharmacol 79: 13–16.
- Amos S, Abbah J, Chindo B, Edmond I, Binda L, Adzu B, Buhari S, Odutola AA, Wambebe C, Gamaniel K (2005): Neuropharmacological effects of the aqueous extract of Nauclea latifolia root bark in rats and mice. J Ethnopharmacol 97: 53–57.
- Asongalem EA, Foyet HS, Ngogang J, Folefoc GN, Dimo T, Kamtchouing P (2004): Analgesic and antiinflammatory activities of Erigeron floribundus. J Ethnopharmacol 91: 301–308.
- Aydin S, Beis R, Can OD (2003): Analgesic and antispasmodic activities of 2-(2-nitro-phenyl)-1H-benzimidazole 5-carboxylic acid: Evidence for the importance of the 2-(o-substituted phenyl) group. Pharmazie 58: 405–408.
- Aydin S, Demir T, Oztürk Y, Başer KH (1999): Analgesic activity of Nepeta italica L. Phytother Res 13: 20–23.
- Baytop T (1999): Therapy with Plants in Turkey (Past and Present). Istanbul, Nobel Medical Bookstore, p. 147.
- Bourin M, Bougerol T, Guitton B, Broutin E (1997): A combination of plant extracts in the treatment of outpatients with adjustment disorder with anxious mood: Controlled study versus placebo. Fund Clin Pharmacol 11: 127–132.
- Calixto JB, Beirith A, Ferreira J, Santos AR, Filho VC, Yunes RA (2000): Naturally occurring antinociceptive substances from plants. Phytother Res 14: 401–418.
- Chang Q, Zuo Z, Harrison F, Chow MSS (2002): Hawthorn. J Clin Pharmacol 42: 605–612.
- Coelho LP, Reis PA, de Castro FL, Gayer CR, da Silva Lopes C, da Costa e Silva MC, de Carvalho Sabino KC, Todeschini AR, Coelho MG (2005): Antinociceptive properties of ethanolic extract and fractions of Pterodon pubescens Benth. seeds. J Ethnopharmacol 98: 109–116.
- D’Amour FE, Smith DL (1941): A method for determining loss of pain sensation. J Pharmacol Exp Ther 72: 74–79.
- de Fátima Arrigoni-Blank M, Dmitrieva EG, Franzotti EM, Antoniolli AR, Andrade MR, Marchioro M (2004): Anti-inflammatory and analgesic activity of Peperomia pellucida (L.) HBK (Piperaceae). J Ethnopharmacol 91: 215–218.
- Della Loggia R, Tubaro A, Redaelli C (1981): Evaluation of the activity on the mouse CNS of several plant extracts and a combination of them. Riv Neurol 51: 297–310.
- Dos Santos JG Jr, Blanco MM, Do Monte FH, Russi M, Lanziotti VM, Leal LK, Cunha GM (2005): Sedative and anticonvulsant effects of hydroalcoholic extract of Equisetum arvense. Fitoterapia 76: 508–513.
- Dos Santos MD, Almeida MC, Lopes NP, de Souza GE (2006): Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol Pharm Bull 29: 2236–2240.
- Duke AJ (2002): Handbook of Medicinal Herbs. London, CRS Press. pp 367–369.
- European Pharmacopoeia Commission (2005): European Pharmacopoeia. Strasbourg, Council of Europe, pp. 1712–1713.
- Fiore M, Alleva E, Moroni R, Aloe L (1998): Infection with Schistosoma mansoni in mice induces changes in nociception and exploratory behavior. Physiol Behav 65: 347–353.
- Gabra BH, Sirois P (2003): Beneficial effect of chronic treatment with the selective bradykinin B1 receptor antagonists, R-715 and R-954, in attenuating streptozotocin-diabetic thermal hyperalgesia in mice. Peptides 24: 1131–1139.
- Garcia MD, Saenz MT, Ahumada MC, Cert A (1997): Isolation of three triterpenes and several aliphatic alcohols from Crataegus monogyna. J Chromatogr A 767: 340–342.
- Gomes NM, Rezende CM, Fontes SP, Matheus ME, Fernandes PD (2007): Antinociceptive activity of Amazonian copaiba oils. J Ethnopharmacol 109: 486–492.
- Gülçin I, Küfrevioglu OI, Oktay M, Büyükokuroglu ME (2004): Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol 90: 205–215.
- Harborne JB, Williams CA (2000): Advances in flavonoid research since 1992. Phytochemistry 55: 481–504.
- Iwamoto ET (1981): Locomotor activity and antinociception after putative mu, kappa and sigma opioid receptor agonists in the rat: Influence of dopaminergic agonists and antagonists. J Pharmacol Exp Ther 217: 451–460.
- Kang TH, Jeong SJ, Kim NY, Higuchi R, Kim YC (2000): Sedative activity of two flavonol glycosides isolated from the flowers of Albizzia julibrissin Durazz. J Ethnopharmacol 71: 321–323.
- Kaplancikli ZA, Turan-Zitouni G, Ozdemir A, Devrim Can O, Chevallet P (2009): Synthesis and antinociceptive activities of some pyrazoline derivatives. Eur J Med Chem 44: 2606–2610.
- Kekesi G, Dobos I, Benedek G, Horvath G (2003): Antinociceptive activity of Sempervivum tectorum L. extract in rats. Phytother Res 17: 1032–1036.
- Koster R, Anderson M, De Beer EJ (1959): Acetic acid and analgesic screening. Fed Amer Soc Exp Biol 18: 412–415.
- Maleki-Dizaji N, Fathiazad F, Garjani A (2007): Antinociceptive properties of extracts and two flavonoids isolated from leaves of Danae racemosa. Arch Pharmacol Res 30: 1536–1542.
- Mills S, Bone K (2000): Principles and Practice of Phytotherapy: Modern Herbal Medicine. Edinburgh, Churchill Livingstone, pp 439.
- Morgan MM, Fossum EN, Stalding BM, King MM (2006): Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain 7: 358–366.
- Narita M, Suzuki T, Misawa M, Nagase H (1993a): Antagonism of the morphine-induced Straub tail reaction by kappa-opioid receptor activation in mice. Psychopharmacol 110: 254–256.
- Narita M, Takahashi Y, Takamori K, Funada M, Suzuki T, Misawa M, Nagase H (1993b): Effects of kappa-agonist on the antinociception and locomotor enhancing action induced by morphine in mice. Japan J Pharmacol 62: 15–24.
- Orhan I, Özcelik B, Kartal M, Özdeveci B, Duman H (2007): HPLC quantification of vitexine-2″-O-rhamnoside and hyperoside in three Crataegus species and their antimicrobial and antiviral activities. Chromatographia 66: S153-S157.
- Ozcan M, Haciseferogullari H, Marakoglu T, Arslan D (2005): Hawthorn (Crataegus spp.) fruit: some physical and chemical properties. J Food Eng 69: 409–413.
- Ozturk N, Baser KH, Aydin S, Ozturk Y, Calis I (2002): Effects of Gentiana lutea ssp. symphyandra on the central nervous system in mice. Phytother Res 16: 627–631.
- Pérez-Ortega G, Guevara-Fefer P, Chávez M, Herrera J, Martínez A, Martínez AL, González-Trujano ME (2008): Sedative and anxiolytic efficacy of Tilia americana var. mexicana inflorescences used traditionally by communities of State of Michoacan, Mexico. J Ethnopharmacol 116: 461–468.
- Pietta P, Manera E, Ceva P (1986): Isocratic liquid chromatographic method for the simultaneous determination of Passiflora incarnata L. and Crataegus monogyna flavonoids in drugs. J Chromatogr 357: 233–237.
- Pittler MH, Guo R, Ernst E (2008): Hawthorn extract for treating chronic heart failure. Cochrane Database Syst Rev 1: CD005312.
- Pittler MH, Schmidt K, Ernst E (2003): Hawthorn extract for treating chronic heart failure: Meta-analysis of randomized trials. Am J Med 114: 665–674.
- Rigelsky JM, Sweet BV (2002): Hawthorn: Pharmacology and therapeutic uses. Am J Health-Syst Pharm 59: 417–422.
- Schmauss C, Yaksh TL (1984): In vivo studies on spinal receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther 228: 1–12.
- Shinde UA, Phadke AS, Nair AM, Mungantiwar AA, Dikshit VJ, Saraf MN (1999): Studies on the anti-inflammatory and analgesic activity of Cedrus deodara (Roxb.) Loud. wood oil. J Ethnopharmacol 65: 21–27.
- Soulimani R, Younos C, Jarmouni S, Bousta D, Misslin R, Mortier F (1997): Behavioural effects of Passiflora incarnata L. and its indole alkaloid and flavonoid derivatives and maltol in the mouse. J Ethnopharmacol 57: 11–20.
- Takeda H, Tsuji M, Matsumiya T (1998): Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol 350: 21–29.
- Thirugnanasambantham P, Viswanathan S, Mythirayee C, Krishnamurty V, Ramachandran S, Kameswaran L (1990): Analgesic activity of certain flavone derivatives: A structure-activity study. J Ethnopharmacol 28: 207–214.
- Thirugnanasambantham P, Viswanathan S, Ramaswamy S, Krishnamurthy V, Mythirayee CI, Ramachandran S, Kameswaran L (1988): Involvement of calcium in flavonoid analgesia. Eur J Pharmacol 152: 367–369.
- Viana GSB, Bandeira MAM, Moura LC, Souza-Filho MVP, Matos FJA, Ribeiro RA (1997): Analgesic and antiinflammatory effects of the tannin fraction from Myracrodruon urundeuva Fr. All. Phytother Res 11: 118–122.
- Vonvoigtlander PF, Lahti RA, Ludens JH (1983): U-50,488: A selective and structurally novel non-mu (kappa) opioid agonist. J Pharmacol Exp Ther 224: 7–12.
- Votava M, Hess L, Slíva J, Krsiak M, Agová V (2005): Dexmedetomidine selectively suppresses dominant behavior in aggressive and sociable mice. Eur J Pharmacol 523: 79–85.
- Wong CH, Day P, Yarmush J, Wu W, Zbuzek UK (1994): Nifedipine-induced analgesic after epidural injections in rats. Anesth Anal 79: 303–306.