Abstract
The present study was conducted to evaluate the antioxidant and anti-inflammatory activities of Jungia paniculata (DC.) A. Gray (Asteraceae), used traditionally in Peru. The dry leaves were extracted with methanol, 50% methanol, and water. The anti-inflammatory activity of this plant was studied using in vitro (nitric oxide production in RAW 264.7 macrophages and sPLA2 inhibition assay) and in vivo (carrageenan-induced paw edema in rats and TPA-induced ear edema in mice) model systems. The antioxidant activity of extracts was studied using three in vitro model systems (DPPH• radical-scavenging assay, ABTS•+ assay, and superoxide radical-scavenging activity). The results have been correlated with total phenolics and total flavonoids contents. In the NO test of the extracts of Jungia paniculata, no significant cytotoxicities were observed at the concentrations determined by MTT assay. Only the MeOH50 extract of Jungia paniculata significantly inhibited PLA2 enzyme activity (82.3 ± 2.6%). At 3 h, the 50% methanol extract of Jungia paniculata at an oral dose of 500 mg/kg showed significant suppression of carrageenan-induced rat paw edema (36.36%). The same extract induced a 93.99% reduction in TPA-induced edema in topical administration. The extracts exhibited a high antioxidant activity and contained high total levels of polyphenols and flavonoids. There was a significant linear correlation between total phenolics and flavonoids contents and antioxidant activity in the three models used. In conclusion, Jungia paniculata possesses anti-inflammatory and antioxidant properties, which confirm the use of this plant in folk medicine as a topical anti-inflammatory herbal.
Introduction
Free radicals and reactive oxygen species (ROS) are well known inducers of cellular and tissue pathogenesis, leading to several human diseases such as cancer and inflammatory disorders, as well as in the aging process (CitationHalliwell, 1994; CitationAviram, 2000). Antioxidants provide protection to living organisms from damage caused by uncontrolled production of ROS and the concomitant lipid peroxidation, protein damage, and DNA strand breaking (CitationGhosal et al., 1996). Several anti-inflammatory, digestive, antinecrotic, neuroprotective, and hepatoprotective drugs have recently been shown to have an antioxidant and/or radical scavenging mechanism as part of their activity (CitationLin et al., 2000; CitationRepetto & Lesuy, 2002). The use of traditional medicine is widespread, and plants still represent a large source of natural antioxidants that might serve as leads for the development of novel drugs (CitationPerry et al., 1999).
Jungia paniculata (DC.) A. Gray (Asteraceae) is an herbaceous plant widespread in the West Peruvian Andes between 2500 and 3500 m of altitude. This plant, commonly called caramati, is used in traditional medicine as an anti-inflammatory and genitourinary anti-infective (CitationDe Feo, 1992). Our ethnopharmacological studies on this plant in Nor-Yauyos (Peru) showed that leaves of Jungia paniculata applied directly on the edema site during 24 h caused a reduction of inflammation. Also, the topical administration of infusions with water has been employed for genitourinary infections (CitationAllende et al., 2000).
From phytochemical studies, it is recognized that Jungia paniculata contains two flavonol glycosides (CitationD’Agostino et al., 1995), but a survey of the literature did not find any scientific publication on biological activities or constituents of this plant, including reports on toxicity.
In this study, anti-inflammatory and antioxidant activities were selected to prove and validate the traditional use of Jungia paniculata. Three different polar extracts were prepared and tested. The anti-inflammatory activity of this plant was studied using in vitro and in vivo model systems. In order to reduce the number of animals for testing, the putative anti-inflammatory activity was preliminarily screened in vitro, and only the most effective extract was further studied for anti-inflammatory activity in vivo. The antioxidant activity of Jungia paniculata (leaves) extracts was studied using three in vitro model systems, and the results have been correlated with total phenolics and total flavonoids contents.
Materials and methods
Chemicals and biochemicals
All chemicals and biochemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Panreac (Barcelona, Spain).
Plant material and preparation of the extracts
Leaves of Jungia paniculata were collected in May 2001 (Peru), and Luz Amanda Vivas (Agronomist Engineer) identified the species. A voucher specimen (Number 43) is deposited in the Herbarium of the Rural Valle Grande Institute, San Vicente de Cañete (Peru). The leaves were dried at room temperature. The pulverized material (500 g) was macerated at room temperature with different solvents (2.5 L each): methanol (MeOH), 50% methanol (MeOH50), and water (H2O), and 500 mL portions of each underwent daily agitation for 2 weeks. The portions of each solvent were combined and taken to dryness by a rotary evaporator. Each fraction of Jungia paniculata thus obtained was kept in a well-closed container in a refrigerator until use.
Phytochemical screening
Phytochemical screening of the powdered leaves for the presence of alkaloids was performed with Mayer’s and Dragendorff’s reagents, flavonoids with the use of Mg and HCl, tannins with 1% gelatin and 10% NaCl solutions, cardiac glycosides with FeCl2 and H2SO4, cyanogenic glycosides with picrate paper, terpenoids with the Liebermann–Burchard method and with H2SO4, anthraquinones using Borntrager’s reaction, and saponins using the aphrometric index (CitationTrease & Evans, 1983; CitationBruneton, 2000).
HPLC-fingerprint analysis
High performance liquid chromatography (HPLC) (Waters W600; Milford, MA, USA) was used to analyze the extracts of Jungia paniculata. Chromatography was performed on a C-18 reversed-phase column (Nova-Pak; 150 mm × 3.9 mm., 4 µm). Detection was in the range 210–500 nm. The column temperature was maintained at 25°C. The dried extract (10 mg) was dissolved in 1 mL of the corresponding solvent using a subsonic bath, followed by filtration of the solution over a Millipore® filtration unit, type HV, 0.45 µm, for subsequent HPLC-ultraviolet (UV) determination. The gradient was formed by varying the proportions of acetonitrile (A) and water (B) containing 0.5% (v/v) acetic acid. The elution system was: 0–5 min, 90–80% of B; 5–20 min, 80–20% of B; 20–25 min, 20–90% of B. The flow rate employed was 1.0 mL/min throughout the run. The injected volume of sample was 10 µL.
Anti-inflammatory activity
Cell culture
Murine RAW 264.7 macrophages were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 µg/mL streptomycin, all from Invitrogen (Langley, OK, USA), in an incubator at 37°C in a humidified atmosphere of 95% air and 5% CO2. Macrophages were removed from the tissue culture flask with the aid of a cell scraper. Cells were then cultured in dishes at a density of 5 × 105 cells/mL and, when sub-confluent, the medium was substituted by DMEM supplemented with 0.5% fetal bovine serum. The cells were then subjected to the treatment described below.
Cytotoxicity
The cytotoxicity of the extracts was measured with the aid of Mosmann’s colorimetric assay (CitationMosmann, 1983). First, RAW 264.7 cells (5 × 105 per mL) were exposed to the extract (final concentration 0.5–44.6 µg/mL; final volume 200 µL) in a 96-well microplate for 24 h. Then the culture medium was eliminated, and 100 µL per well of a 0.5 mg/mL solution of 3-(4,5-dimethylthiazolyl)-2,5-diphenyl-tetrazolium bromide (MTT; Sigma-Aldrich) was added. The microplates were then incubated at 37°C until blue deposits were visible. The supernatant was eliminated and the colored metabolite was dissolved in dimethylsulfoxide (Sigma-Aldrich; 100 µL per well). This reaction was performed in triplicate. Absorbance was measured at 570 nm with the aid of a Labsystems Multiskan MCC/340 plate reader (Helsinki, Finland).
Nitric oxide production in RAW 264.7 macrophages
RAW 264.7 murine macrophages were cultured in DMEM medium containing 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% fetal bovine serum. Cells were removed from the tissue culture flask with a cell scraper and resuspended until there was a final concentration of 5 × 105 cells/mL in the culture medium. Macrophages (RAW 264.7) were then co-incubated in a 96-well culture plate (200 µL), with 1 µg/mL (final concentration) of lipopolysaccharide (LPS; Sigma-Aldrich) at 37°C for 24 h in the presence of the test extract (0.5–44.6 µg/mL), dexamethasone (1.8 µg/mL = 5 µM), or vehicle. The presence of nitrites was then determined in the culture supernatant with the aid of Griess reagent (Sigma-Aldrich). Nitrite production was assessed as the index of nitric oxide generation in the induction phase.
Phospholipase A2 activity assay
Secretory phospholipase A2 (sPLA2) was assayed according to a modification of the method of Franson (CitationFranson et al., 1974). Escherichia coli strain CECT 101 was seeded onto medium containing 1% tryptone, 0.5% NaCl, and 0.6% sodium dihydrogen orthophosphate, pH 5.0, and grown for 6–8 h at 37°C in the presence of 5 µCi/mL [3H]oleic acid (specific activity, 10 Ci/mmol). After centrifugation at 2500 g for 10 min, the cells were washed in buffer (0.7 M Tris-HCl, 10 mM CaCl2, 0.1% bovine serum albumin (BSA), pH 8.0), resuspended in saline, and autoclaved for 30–45 min. At least 95% of the radioactivity was incorporated into phospholipids. Human recombinant synovial enzyme was diluted in 10 µL of 100 mM Tris-HCl/1 mM CaCl2 buffer, pH 7.5. Supernatants (10 µL) of exudates from zymosan-injected rat air pouches (CitationPayá et al., 1997) were also used as a source of sPLA2. Enzymes were preincubated at 37°C for 5 min with 2.5 µL of test compound solution or its vehicle in a final volume of 250 µL. Incubation proceeded for 15 min in the presence of 10 µL of autoclaved oleate-labeled membranes, and was terminated by the addition of 100 µL of an ice-cold solution of 0.25% BSA in saline to a final concentration of 0.07%, w/v. After centrifugation at 2500 g for 10 min at 4°C, the radioactivity in the supernatants was determined by liquid scintillation counting.
Animals
For in vivo experiments, groups of 10 male albino Swiss mice (25–30 g each) and male Wistar rats (150–200 g each) from the Center for Applied Pharmacobiology Research (University of Navarra) were used. Housing conditions were five animals per cage at room temperature of 22–23°C with free access to pellet chow, and tap water ad libitum. The animals were allowed to acclimatize for 1 week before experiments started. The Institutional Animal Care and Use Committee of the University of Navarra approved the experimental protocols.
Carrageenan-induced rat paw edema
An edema was induced on the right hind foot of rats by subplantar injection of 0.1 mL of a solution of 3% carrageenan in 1.5% (w/v) saline solution (CitationWinter et al., 1962). The reference group was treated with indomethacin (10 mg/kg, p.o.) (Sigma-Aldrich). A control group received only vehicle. Doses of 250 and 500 mg/kg of Jungia paniculata 50% methanol extract, dissolved in carboxymethylcellulose 1%, were administered orally 0.5 h before carrageenan injection. The volumes of the injected and contralateral paws were measured 1, 2, 3, and 4 h after induction of inflammation using a plethysmometer (Ugo Basile), and the edema was expressed as an increase in paw volume due to carrageenan injection. The anti-inflammatory activity was calculated at each time of observation, as percent inhibition of edema in the animals treated with the substances under test in comparison to control animals.
Mouse ear inflammation induced by TPA
Inflammation was induced through topical application of 2.5 µg of 12-O-tetradecanoylphorbol-13-acetate (TPA; Sigma-Aldrich) dissolved in 20 µL of acetone (Panreac) on the right ear (CitationTonelli et al., 1965). This was applied by an automatic pipette in 10 µL volumes to both anterior and posterior surfaces of the right ear. The left ear (control) received the same volume of acetone. Indomethacin (Sigma-Aldrich) in acetone was used as the reference drug (0.5 mg/ear). The MeOH50 extract of Jungia paniculata was applied in three doses of 2.5 mg/ear every 30 min; the first dose was applied at the beginning of the experiment. The system of successive doses was used in order to work in a similar fashion to that of folk medicine. Inflammation was allowed to develop for 4 h, after which the animals were killed by cervical dislocation and a section of the central portion of both ears was obtained and weighed. Swelling was assessed in terms of the mean weight increase of each ear, while inhibition of swelling was expressed as the weight reduction in comparison to the control group treated with TPA only (CitationTubaro et al., 1985). Details of the protocol have been previously described by CitationCalvo et al. (1998).
Antioxidant activity
DPPH radical-scavenging activity
The antioxidant activity of the extracts was determined in terms of hydrogen-donating or radical-scavenging ability, using the stable radical DPPH• (2,2-diphenyl-1-picrylhydrazyl; Sigma-Aldrich) (CitationBlois, 1958) with slight modification. The assay was carried out using 96-well plates and absorbance was recorded with a PowerWave XS microplate reader (Bio-Tek KCjunior program) at 25°C. A 150 µL aliquot of sample or control was mixed with 150 µL of methanol DPPH• (4 mg/mL) solution. Changes in absorbance of all samples and standards were measured at 517 nm at 30 min. The activity was evaluated according to the equation: radical-scavenging activity (RSA)% = (Ac – As)/Ac × 100%. As and Ac are the absorbances at 517 nm of the reaction mixture with sample and control, respectively. The IC50 values were obtained using the GraphPad Prism v.4 program by nonlinear regression analysis, and denoted the concentration of sample required to scavenge 50% of DPPH• radical. All experiments were repeated at least three times.
ABTS•+ assay
ABTS•+ was prepared by reaction of 7 mM aqueous ABTS solution (2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid; Sigma-Aldrich) and 2.45 mM potassium persulfate solution as proposed (CitationRe et al., 1999). The mixed solution was stored in the dark for 16 h, and the radical cation solution was further diluted in 50% methanol solution until an initial absorbance value of 0.7 at 734 nm was reached. Solutions of Jungia paniculata extracts were prepared in methanol, and 20 µL was pipetted into 96-well plates. Diluted ABTS•+ radical solution (200 µL) was added to each well and the readings were recorded for 6 min at 734 nm using a MicroWave XS microplate reader (Bio-Tek KCjunior program) at 25°C. The IC50 (µg/mL) was determined for every extract using the GraphPad Prism v.4 program. The radical-scavenging activity was calculated using the formula mentioned above for DPPH•. All measurements were conducted in triplicate and results were averaged.
Superoxide anion-scavenging activity
Superoxide radicals were generated by a hypoxanthine/xanthine oxidase (XO) enzymatic system (CitationGuzmán et al., 2001). Superoxide radical-scavenging activity was assayed according to the method of CitationValentao et al. (2001) with some modifications. In the sample wells (96-well microplate) were mixed 75 µL of 145 µM xanthine (Sigma-Aldrich), 75 µL of 50 µM nitroblue tetrazolium chloride (NBT; Sigma-Aldrich), and 75 µL of extract, all dissolved in 50 mM phosphate buffer. The reaction was initiated by adding 75 µL of xanthine oxidase (0.29 unit/mL was dissolved in 50 mM phosphate buffer) (Sigma-Aldrich). The superoxide radical inhibition was measured spectrophotometrically at 560 nm after 2 min of preincubation at room temperature. A MicroWave XS microplate reader (Bio-Tek KCjunior program) at 25°C was used. Estimation of IC50 values, the concentration of sample required to scavenge 50% superoxide to inhibit XO, were determined by monitoring the effect of decreasing concentrations of compounds on the scavenging activity. IC50 values were calculated using the GraphPad Prism v.4 program. All measurements were conducted in triplicate.
Quantification of total phenolics and total flavonoids
The total phenolics composition of Jungia paniculata extracts was quantified spectrophotometrically by the Prince method (CitationPrince & Butler, 1977) with slight modifications, using gallic acid as standard. The assay was carried out using 96-well plates, and absorbance was recorded with the PowerWave XS Microplate Reader (Bio-Tek KCjunior program) at 25°C. A 100 µL aliquot of sample or control was mixed first with 100 µL of 0.004 M FeCl3 (in 0.1 N HCl) solution and finally with 100 µL of 0.36 mM K3Fe(CN)6 solution. Fifteen minutes later the absorbance was measured at 760 nm. Total phenolics content was expressed as gallic acid equivalents in one gram of dry weight plant. The concentrations of extracts were adjusted taking into account their total phenolics content in order to obtain comparable results in the subsequent experiments. All experiments were repeated at least three times.
Total flavonoids were estimated as rutin equivalents. Plant extract (1 mL) in methanol (10 g/L) was mixed with 1 mL aluminum trichloride in ethanol (20 g/L) and diluted with ethanol to 25 mL. The absorption at 415 nm was read after 30 min at 25°C. Blank samples were prepared from 1 mL plant extract and one drop of acetic acid, and diluted to 25 mL. The rutin calibration curve was prepared in methanol solutions using the same procedure. All determinations were carried out in triplicate and the mean values were used.
Statistics
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Dunnett’s test (for comparison of samples with the control group) or Tukey’s test. Differences were considered significant at values of p less than 0.05. Inhibition percentages (%I) were calculated from differences between the extract-treated groups and the control group.
Results and discussion
Jungia paniculata was extracted with methanol, 50% methanol, and water by maceration. Phytochemical screening showed the presence of phenolics, flavonoids, and saponins in all the extracts, while alkaloids, tannins, cardiac glycosides, cyanogenic glycosides, terpenoids, and anthraquinones were absent. The C-18 column and mobile phase used were found to be appropriate for the preliminary characterization of extracts of Jungia paniculata by HPLC-UV. shows HPLC-fingerprint max-plot chromatograms of the three extracts of Jungia paniculata. Chromatographic analysis showed that the three extracts contained flavonoids and phenolic acid derivatives in different proportions.
Figure 1. HPLC-UV analysis of Jungia paniculata with max-plot responses. A, phenolic acid; F, flavonoid.
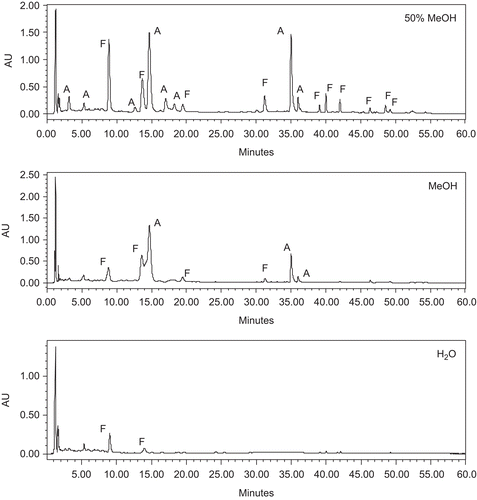
Data of extraction yields were similar for the three solvents employed (). The order of increase of phenolic content in the extracts was: water < 50% methanol < methanol extract (). On the other hand, all the extracts were found to be rich in flavonoids. The 50% methanol extract was found to be the richest in flavonoid content (6.70 ± 0.48%).
Table 1. Extract yield and total phenols and flavonoids contents for Jungia paniculata.
In the literature can be found a high number of publications regarding medicinal plants with correlations between anti-inflammatory/antioxidant activity and phenolic/flavonoid content (CitationSpiteller et al., 2008; CitationYin et al., 2008; CitationAquila et al., 2009; CitationConforti et al., 2009). For this reason, the three extracts of Jungia paniculata leaves were tested for their anti-inflammatory and antioxidant activities using in vitro and in vivo models.
For first information about the anti-inflammatory activity of Jungia paniculata, all the extracts was tested in two different in vitro models, nitric oxide production in RAW 264.7 macrophages and sPLA2 inhibition assay, because these methods were effective and fast for screening extracts with anti-inflammatory activity before using animals.
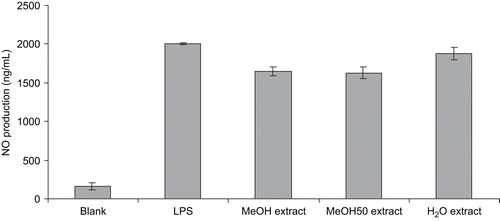
In the present study, all extracts were tested in MTT and nitrite assays to determine cytotoxicities and NO scavenger activities. In the NO test, no significant cytotoxicities were observed at the concentrations used, which were determined by MTT assay (data not shown). The inhibitory effects of the tested extracts on NO production were assessed by LPS-induced RAW 264.7 macrophages, and the results are shown in . When treated with LPS, the nitrite content was notably increased. When the macrophage cells were treated with the extracts, NO production induced by LPS was not significantly reduced. These data led to the hypothesis that extracts from Jungia paniculata did not possess anti-inflammatory activity through NOS (nitric oxide synthase) induction.
Figure 2. Effect of three extracts of Jungia paniculata on LPS-induced NO production in RAW 264.7 macrophage cells. Values are expressed as mean ± SD from three independent experiments.
Phospholipase A2 catalyzes the first step of the production of proinflammatory compounds collectively known as eicosanoids. The generation of proinflammatory eicosanoids involves a series of free radical intermediates with simultaneous release of reactive oxygen species. Reactive oxygen species formed during arachidonic acid metabolism generate lipid peroxides and cytotoxic products such as 4-hydroxynonenal and acrolein, which induce cellular damage. Thus, PLA2 catalyzes the rate-limiting step in the production of proinflammatory eicosanoids and free radicals. These peroxides and reactive oxygen species in turn activate the PLA2 enzyme and further attenuate the inflammatory process. Therefore, scavenging of these free radicals and inhibition of PLA2 enzyme simultaneously by a single molecule such as an antioxidant is of great therapeutic relevance for the development of anti-inflammatory molecules. Many secondary metabolites from plants and marine sponges exhibit both anti-inflammatory and antioxidant properties. In this way, extracts with dual activities may prove to be powerful anti-inflammatory drugs (CitationNanda et al., 2007). Only the MeOH50 extract of Jungia paniculata significantly inhibited PLA2 enzyme activity (82.3 ± 2.6%) with an efficacy comparable to that of SCA (83.8 ± 5.1%) ().
Table 2. Effect of extracts of Jungia paniculata on human synovial sPLA2 activity.
The anti-inflammatory activity of the MeOH50 extract of Jungia paniculata was further confirmed by in vivo tests using two models of edema: carrageenan-induced paw edema in rats and TPA-induced ear edema in mice.
The MeOH50 extract of Jungia paniculata at an oral dose of 500 mg/kg showed a significant suppression of carrageenan-induced rat paw edema compared with the control group (p < 0.05). Indomethacin also showed a clear inhibition of the inflammation induced by carrageenan, compared with the control group (p < 0.05). In control animals, the subplantar injection of carrageenan produced a local edema in the following 30 min that increased progressively to reach a maximal intensity, 3 or 4 h after injection of the phlogistic agent, and then began to decline. Jungia paniculata inhibited the paw edema, with different results according to the dose used. Thus, at 3 h, at the dose of 500 mg/kg there was a 36.36% reduction in edema (0.42 ± 0.06 mL) compared to control (0.66 ± 0.08 mL). The dose of 250 mg/kg of the extract was ineffective in reducing the edema (). The MeOH50 extract was also studied for its anti-inflammatory activity by TPA-induced mouse ear edema. The results for topical application of the extract are shown in . They indicate a significant reduction of the edema induced by TPA after topical application of the MeOH50 extract and the positive control indomethacin, 30 min post-dosing at the dose level tested. Jungia paniculata induced a 93.99% reduction in TPA-induced edema. At the dose used, the anti-inflammatory effect of the extract was higher than that of indomethacin (74.83%). In general, topical administration at the site of inflammation is the most effective treatment because a much higher concentration of the drug can be obtained. This is what occurs with extracts, which are more active when they are administered topically than when they are given orally (CitationRecio et al., 1994). The reason could be related to the ability of extracts to penetrate the skin of the ear. This finding suggests that the MeOH50 extract may be an important therapeutic strategy for the treatment of inflammatory skin diseases.
Table 3. Anti-inflammatory effect of MeOH50 extract of Jungia paniculata on rat paw edema.
Table 4. Anti-inflammatory effect of extracts of Jungia paniculata on mouse ear edema.
Antioxidants can protect cells against the damaging effects of reactive oxygen species. An imbalance between antioxidants and reactive oxygen species results in oxidative stress, finally leading to cellular damage. Oxidative stress has been linked to cancer, aging, atherosclerosis, inflammation, and neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases (CitationGetoff, 2007). Therefore, antioxidants occurring in plants may play a significant role in health protection. The mechanism of antioxidant action can include free radical-scavenging activity and suppression of reactive oxygen species formation, either by inhibition of enzymes or by chelating trace elements (CitationVan Acker et al., 1996). On the other hand, the response of antioxidants depends on factors such as the solvent and substrate used in a test and affinity between the substrate and antioxidant. For this reason, a variety of in vitro techniques have been developed. Hence, it is better to use different assays based on different mechanisms to evaluate the antioxidant capacity (CitationWangensteen et al., 2004; CitationMoure et al., 2006). In the present work, three different methods were successfully used for evaluation of the antioxidant activity of the crude extracts: DPPH radical-scavenging assay, ABTS•+ assay, and superoxide radical-scavenging activity (enzymatic assay) systems. The results were compared with butylhydroxytoluene (BHT), one of the most employed synthetic antioxidants.
Both DPPH• and ABTS•+ radicals have been used extensively to evaluate the antioxidant properties of natural products. These reactions have been widely used to investigate the ability of plant extracts and fractions and/or pure compounds of these to act as free radical scavengers or hydrogen donors. All the extracts were capable of scavenging the DPPH• radical. The activities of the 50% methanol (11.91 ± 0.55 µg/mL) and methanol (19.48 ± 1.15 µg/mL) extracts showed them to be the most potent scavengers. The second method, the ABTS•+ assay, used to measure antioxidant activity revealed similar results to the DPPH• assay. The results of both assays reveal that 50% methanol and methanol extracts are capable of scavenging free radicals and may prevent initiation of free radical-mediated chain reactions by preventing the abstraction of hydrogen from susceptible polyunsaturated fatty acids. Superoxide anions were generated in vitro enzymatically by the hypoxanthine/xanthine oxidase system that reduces NBT and forms a chromophore, diformazan. In this system, there are two possibilities: either the plant extract may scavenge the O2•− or it may inhibit the xanthine oxidase activity. In this study, the effect of plant extracts on xanthine oxidase activity was evaluated, and the IC50 values for its inhibition appear in . The 50% methanol and methanol extracts were inhibitors of xanthine oxidase (6.53 ± 0.04 µg/mL and 8.67 ± 0.42 µg/mL, respectively).
Table 5. Free radical scavenging and antioxidant enzyme activities in extracts of Jungia paniculata.
The antioxidant effect of natural components has previously been studied in relation to the prevention of coronary diseases, cancer, age-related degenerative brain disorders, and inflammatory disorders (CitationGilani et al., 2000; CitationMiyamoto et al., 2007; CitationStevenson & Hurst, 2007). Recent investigations on the antioxidant activity of plants show that polyphenols are the carriers of these properties (CitationChen & Ho, 1997; CitationFerrari, 2000; CitationBors & Stettmaier, 2001; CitationYizhong et al., 2004). Probably the most important natural phenolics are flavonoids because of their broad spectrum of chemical and biological activities, including radical-scavenging properties (CitationKähkönen et al., 2001). Significant (p < 0.05) negative correlation was observed between total phenolics content and the IC50 for DPPH• radical-scavenging activity (r2 = –0.885), ABTS•+ radical-scavenging activity (r2 = –0.848), and superoxide anion radical-inhibition activity (r = –0.855). Results showed that total flavonoids content had an extremely high (p < 0.01) correlation with DPPH• radical-scavenging activity (r2 = –0.946), ABTS•+ radical-scavenging activity (r2 = –0.967), and superoxide anion radical-inhibition activity (r2 = –0.958). Therefore, good correlation suggests that phenolic compounds, mainly flavonoids, present in Jungia paniculata leaves play an important role as antioxidants.
Conclusions
The 50% methanol extract of Jungia paniculata exhibited significant antioxidant and anti-inflammatory properties, confirming the validity of the local use of this plant for medicinal purposes. The results lead to the hypothesis that Jungia paniculata possesses anti-inflammatory activity through inhibition of the PLA2 enzyme. Further studies need to be performed to determine the precise mechanism of action and to identify the chemical components present in the extract.
Declaration of interest
This investigation was supported by the Asociación de Amigos (Universidad de Navarra), Departamento de Educación y Cultura (Gobierno de Navarra), and Plan de Investigación (Universidad de Navarra).
References
- Allende M, Calvo J, Fernández de la Vega M, Gil I, Sasal N, Uriarte I (2000): Manual de plantas medicinales de Nor-Yauyos. Cañete, Peru, Instituto Rural Valle Grande, pp. 21.
- Aquila S, Giner RM, Recio MC, Spegazzini ED, Ríos JL (2009): Anti-inflammatory activity of flavonoids from Cayaponia tayuya roots. J Ethnopharmacol 121: 333–337.
- Aviram M (2000): Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic Res 33: S85–S97.
- Blois MS (1958): Antioxidant determinations by the use of a stable free radical. Nature 181: 1199–1200.
- Bors CM, Stettmaier K (2001): Structure-activity relationships governing antioxidant capacities of plant polyphenols. Methods Enzymol 335: 166–180.
- Bruneton J (2000): Farmacognosia, Plantas Medicinales, 2nd ed. Zaragoza, Spain, Acribia.
- Calvo MI, Vilalta N, Julian AS, Fernández M (1998): Anti-inflammatory activity of leaf extract of Verbena officinalis L. Phytomedicine 5: 465–467.
- Chen JH, Ho CT (1997): Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J Agric Food Chem 45: 2374–2378.
- Conforti F, Rigano D, Menichini F, Loizzo MR, Senatore F (2009): Protection against neurodegenerative diseases of Iris pseudopumila extracts and their constituents. Fitoterapia 80: 62–67.
- D’Agostino M, Senatore F, De Feo V, De Simone F (1995): Flavonol glycosides from Jungia paniculata. Fitoterapia 66: 283–284.
- De Feo V (1992): Medicinal and magical plants in the northern Peruvian Andes. Fitoterapia 63: 417–440.
- Ferrari CKB (2000): Free radicals, lipid peroxidation and antioxidants in apoptosis: implications in cancer, cardiovascular and neurological diseases. Biologia 55: 579–588.
- Franson R, Patriarca P, Elsbach P (1974): Phospholipid metabolism by phagocytic cells. Phospholipase A2 associated with rabbit polymorphonuclear leukocyte granules. J Lipid Res 15: 380–388.
- Getoff N (2007): Anti-aging and aging factors in life. The role of free radicals. Radiat Phys Chem 76: 1577–1586.
- Ghosal A, Sadrieh N, Levin W, Thomas PE (1996): Induction of the male-specific cytochrome P450 3A2 in female rats by phenytoin. Arch Biochem Biophys 332: 153–162.
- Gilani AH, Aziz N, Ali SM, Saeed M (2000): Pharmacological basis for the use of peach leaves in constipation. J Ethnopharmacol 73: 87–93.
- Guzmán S, Gato A, Calleja JM (2001): Anti inflammatory, analgesic and free radical scavenging activities of the marine microalgae Chlorella stigmatophora and Phaeodactylum tricorntum. Phytother Res 15: 224–230.
- Halliwell B (1994): Free-radicals and antioxidants. A personal view. Nutr Rev 52: 253–265.
- Kähkönen MP, Hopia AI, Heinonen M (2001): Berry phenolics and their antioxidant activity. J Agric Food Chem 49: 4076–4082.
- Lin JK, Chen PC, Ho CT, Lin-Shiau SY (2000): Inhibition of xanthine oxidase and suppression of intracellular reactive oxygen species in HL-60 cells by theaflavin-3,3′-digallate, (−)-epigallo-catechin-3-gallate, and propyl gallate. J Agric Food Chem 48: 2736–2743.
- Miyamoto Y, Yamauchi J, Chan JR, Okada A, Tomooka Y, Hisanaga S, Tanoue A (2007): Cdk5 regulates differentiation of oligodendrocyte precursor cells through the direct phosphorylation of paxillin. J Cell Biol 120: 4355–4366.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63.
- Moure A, Sineiro J, Domínguez H, Parajó JC (2006): Functionality of oilseed protein products: A review. Food Res Int 39: 945–963.
- Nanda BL, Nataraju A, Rajesh R, Rangappa KS, Shekar MA, Vishwanath BS (2007): PLA(2) mediated arachidonate free radicals: PLA(2) inhibition and neutralization of free radicals by anti-oxidants – a new role as anti-inflammatory molecule. Curr Top Med Chem 7: 765–777.
- Payá M, García-Pastor P, Coloma J, Alcaraz MJ (1997): Nitric oxide synthase and cyclo-oxygenase pathways in the inflammatory response induced by zymosan in the rat air pouch. Br J Pharmacol 120: 1445–1452.
- Perry E, Walker M, Grace J, Perry R (1999): Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci 22: 273–280.
- Prince ML, Butler LG (1977): Rapid visual estimation and spectrophotometric determination of tannin content of Sorghum grain. J Agric Food Chem 25: 1268–1273.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999): Antioxidant activity applying an improved ABTS•+ radical cation decolourization assay. Free Radic Biol Med 26: 1231–1237.
- Recio MC, Giner RM, Mañez S, Rios JL (1994): Structural considerations on the iridoids as anti-inflammatory agents. Planta Med 60: 232–234.
- Repetto M, Lesuy S (2002): Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res 35: 523–534.
- Spiteller M, Özen T, Smelcerovic A, Zuehlke S, Mimica-Dukić N (2008): Phenolic constituents and the in vitro antioxidant activity of the flowers of Hypericum venustum. Fitoterapia 79: 191–193.
- Stevenson D, Hurst R (2007): Polyphenolic phytochemicals – just antioxidants or much more? Cell Mol Life Sci 64: 2900–2916.
- Tonelli G, Thibault L, Rungler J (1965): A bioassay for the concomitant assessment of the antiphlogistic and thymolytic activities of topically applied corticoids. Endocrinology 27: 625–634.
- Trease GE, Evans WC (1983). Pharmacognosy. London, Bailliere Tindall Press.
- Tubaro A, Dri P, Delbello G, Zilli C, Della Loggia R (1985): The croton oil ear test revisited. Agent Act 17: 347–349.
- Valentao P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, Bastos ML (2001): Antioxidant activity of Centaurium erythraea infusion evidenced by its superoxide radical scavenging and xanthine oxidase inhibitory activity. J Agric Food Chem 49: 3476–3479.
- Van Acker SABE, Van Den Berg D, Tromp MNJL, Griffioen DH, Van Bennekom WP, Van Der Vijgh WJF, Bast A (1996): Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med 20: 331–342.
- Wangensteen H, Samuelsen AB, Malterud KE (2004): Antioxidant activity in extracts from coriander. Food Chem 88: 293–297.
- Winter CA, Risley EA, Nuss GW (1962): Carrageenan-induced edema in the hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111: 544–547.
- Yin Y, Gong F, Wu X, Sun Y, Li Y, Chen T, Xu Q (2008): Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J Ethnopharmacol 120: 1–6.
- Yizhong L, Qiong L, Mei S, Harold C (2004): Antioxidant activity and phenolic compounds of traditional 112 Chinese medicinal plants associated with anticancer. Life Sci 74: 2151–2184.
